Robotic mitral valve replacement; results from the world’s largest series
Introduction
Minimal invasiveness in cardiac surgery has gained wide acceptance over the last few decades. Robotic techniques enable mitral valve surgery to be performed by the least invasive method, with favorable outcomes. The benefit of a robotic approach in mitral valve surgery has already been demonstrated in mitral valve repair procedures. However, similar benefits can also be obtained in mitral valve replacement (MVR) procedures using the robotic approach (1,2).
This study evaluates the clinical outcome of patients with robotic MVR.
Methods
Between January 2010 and April 2022, 117 consecutive patients underwent robotic MVR with or without additional cardiac procedures. All procedures were completed by a single surgical team with Da Vinci Robotic Systems.
Data collection & statistical analysis
Perioperative variables, demographics and early clinical outcomes were prospectively recorded. Categorical parameters are presented as count and percentage while continuous parameters are presented as mean ± standard deviation.
Surgical technique
Robotic surgery was carried out using the Da Vinci XI or SI systems. Patients with mitral valve disease who required valve replacement (with or without an additional cardiac procedure) due to the disease pathology were included. Reoperative mitral valve operations were excluded.
Patients with advanced age with concomitant coronary artery disease or peripheral arterial disease underwent routine preoperative computed tomographic angiography before the operation. Aorta and peripheral branches were assessed using the mean values of peripheral arterial disease, calcification or thrombotic lesions. Peripheral cannulation was mostly performed using the right groin. Our setup for peripheral cannulation was described previously (3,4). When feasible, the right common femoral artery and left femoral vein were cannulated percutaneously under ultrasound and transesophageal echocardiography guidance. Two Proglide suture systems were used for the arterial cannulation, these were implanted before the placement of the arterial cannula with a perpendicular position to each other. In patients with extensive atherosclerotic femoral arteries or plaque/thrombus formation at the level of abdominal or thoracic aorta, we cannulated the axillary artery with surgical exposure.
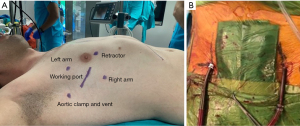
Operative set-up was described earlier (3,4). Following the anesthetic preparation, a mini-thoracotomy, usually 4 cm, at the right 4th intercostal space is performed. Usually, the 3rd and 5th intercostal spaces are used for the insertion of left and right working ports respectively. The Chitwood clamp and a suction vent is placed through a stab incision at the 2nd intercostal space in the anterior axillary line. A soft tissue retractor is placed after the port implantation. The camera is placed through the soft tissue retractor and cardiopulmonary bypass (CPB) is initiated. In patients with severe calcifications at the level of ascending aorta we performed the operation under hypothermic fibrillation without cross clamping (Figure 1). This technique was described elsewhere (5).
The commencement of CPB is followed by opening of the pericardium, classically with an incision of 2 cm anterior and parallel to the phrenic nerve, and further fixation externally. A Chitwood aortic clamp is inserted in the direction of the transverse sinus. Cardiac arrest is established with cross clamp and antegrade cardioplegia delivered through a temporary needle in the ascending aorta through the soft tissue retractor. The preferred cardioplegia solution was Custodiol HTK Solution which was switched to modified Del Nido Solution for all robotic mitral cases after 2020.
Left atriotomy is performed and the left atrial vent and retractor are placed. Since the etiology is mostly rheumatic in patients who underwent robotic MVR, the leaflets are usually thickened and the commissures are often fused. The tissue is gently grabbed with a prograsper and excised using curved scissors or cautery. No additional instruments were used for the excision of the tissue. Contribution from the surgeon on the patients’ side was not feasible for this step. No scoring of the tissue calcification was done. The only exclusion criterion regarding the tissue characteristics was extensive mitral annular calcification. Concomitant procedures are usually performed at this stage. The valve sutures are than placed. Usually, 12–14 pledgeted sutures are implanted at the atrial side and passed through the annulus of the valve prosthesis. These sutures are secured with an automatic mechanical knot fastening system (Cor-Knot, LSI Solution, Victor, NY, USA) (Figure 2).
The atriotomy is closed, a vent is left in place. The aortic needle site is sutured, the needle is placed again for deairing. After deairing the cross clamp is released. The valve prosthesis is assessed using transesophageal echocardiogram guidance. Venting needle is than removed and suture is tightened using a knot pusher. Hemostasis is achieved and the robotic arms are removed. Afterwards, the femoral and jugular venous cannulas are removed. The arterial cannula is removed and the Proglide sutures are tightened.
Between January 2010 and April 2022, 117 consecutive patients underwent robotic MVR. The demographic and operative data are presented in Table 1. Mean age and EuroScore of the patients were 57.1±12.9 and 5.1±5.7, respectively.
Table 1
| Variables | N=117 |
|---|---|
| Age (years) | 57.1±12.9 |
| Female gender | 91 (77.8) |
| NYHA class 3 | 43 (36.8) |
| NYHA class 4 | 2 (1.7) |
| BSA | 1.84±0.19 |
| Diabetes mellitus | 21 (17.9) |
| Hypertension | 32 (27.4) |
| Chronic obstructive pulmonary disease | 7 (6.0) |
| Peripheral artery disease | 11 (9.4) |
| Cerebrovascular disease | 11 (9.4) |
| Neurological dysfunction | 0 (0) |
| Atrial fibrillation | 53 (45.3) |
| LVEF ≤50% | 12 (10.3) |
| EuroScore II | 5.1±5.7 |
| Urgent surgery | 7 (6.0) |
| Cardiac procedure | |
| Isolated valve replacement | 55 (47.0) |
| Valve replacement + combined procedures | 62 (53.0) |
| CPB duration (min) | 143±54 |
| CC time (min) | 93±37 |
Data are expressed as mean ± standard deviation or number (%). NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; CC, cross clamp; BSA, body surface area.
Results
Isolated MVR was performed in 55 (47.0%) patients and combined additional (one or more) cardiac procedures were performed in 62 (53.0%) patients. Additional procedures included; ablation for atrial fibrillation, tricuspid valve replacement, tricuspid valve repair, left atrial appendage ligation, patent foramen ovale closure, left atrial thrombectomy and septal myectomy for hypertrophic obstructive cardiomyopathy. The most common concomitant procedure was ablation for atrial fibrillation with 53 (45.3%) patients. Concomitant tricuspid valve surgery was performed in 25 (21.4%) patients. Within this group tricuspid repair was performed in 21 (17.9%) and tricuspid valve replacement was performed in four (3.4%) of them. Mean CPB time and cross clamp time were 143±54 and 93±37 minutes, respectively. Mean intensive care unit stay time was 26.5±26.0 hours. Postoperative stroke was observed in one (0.9%) patient and new onset renal failure was observed in two (1.7%) patients. Perioperative and postoperative early mortality was observed in three (2.6%) patients. The expected mortality was 5.1%. Postoperative clinical outcome data is presented in Table 2.
Table 2
| Outcomes | N=117 |
|---|---|
| Chest tube output (mL) | 337±349 |
| Reoperation | 0 (0) |
| Mechanical ventilation time (h) | 9.5±19.2 |
| ICU stay time (h) | 26.5±26.0 |
| Postoperative stroke | 1 (0.9) |
| Postoperative dialysis | 2 (1.7) |
| Reintubation | 5 (4.3) |
| 30-day mortality | 3 (2.6) |
| ICU readmission | 6 (5.1) |
| Hospital readmission | 2 (1.7) |
Data are expressed as mean ± standard deviation or number (%). ICU, intensive care unit.
Discussion
Although the majority of the literature data for minimally invasive or robotic mitral surgery focuses on mitral valve repair, there is still a group of patients who require valve replacement (2). Current robotic technology enables safe MVR, with MVR operations accounting for nearly 30% of patients within our robotic program.
Our operative setup is similar with the mitral valve repair procedures. One point which might be assessed as a difference between the valve repair procedures is that the patients who require replacement usually have a history of rheumatic disease. In these patients, the valve and subvalvular tissue, sometimes even the annulus, is much more thickened or calcified. Thus, handling and resecting the tissues might be challenging. The limited strength of robotic scissors should be kept in mind when dealing with a thickened and calcified tissue. Using the cautery might be an option in such cases, however the determination of the extension of the calcifications can be achieved with a preoperative computed tomography. Extensive calcifications should be referred to as an absolute contraindication for robotic MVR (3).
Severe peripheral artery disease and aortic calcifications can be evaluated as a relative contraindication. However, a careful preoperative angiography with computed tomography may help for operative planning. Usually, the axillary arteries are suitable for arterial cannulation in such patients. Ascending aortic calcification is absolute contraindication for aortic cross clamping, but the robotic procedure can be performed without cross clamping under hypothermic fibrillation.
Mean CPB and cross clamp times are around 140 and 90 minutes respectively in this MVR group. However, it should be kept in mind that the majority of patients had one or more additional procedures, such as ablation with left atrial appendage ligation or tricuspid valve repair or replacement. Usually, this group of patients are exposed to prolonged rheumatic disease and the rate of atrial fibrillation and accompanying tricuspid valve disease is detected in a higher rate. Our CPB and cross clamp times are usually below 90 and 60 minutes respectively in an isolated robotic MVR procedure.
Another point regarding our technique that should be evaluated can be the percutaneous approach for cannulation. After the launch of this method in our clinic for the last several years, we have observed a zero groin complication rate postoperatively. Keeping in mind that the risk profile of our patients has dramatically increased in the last decade, the efforts for preventing any kind of operative complication should be accepted as a must. This percutaneous approach is especially beneficial in obese patients and enables a safe peripheral cannulation (5,6).
Different surgical teams have reported good clinical outcomes even in the long term, demonstrating the feasibility and safety of robotic MVR (7-9). In one of the latest and largest series with a long term follow up, the CPB and aortic cross-clamping times were reported as 122.02±25.45 and 85.68±20.70 minutes, respectively without any operative mortality. Thus, we may conclude that this technique may be reproducible and beneficial for patients requiring MVR.
Our robotic mitral valve program has been active since 2010. Before starting this program, the team, including two surgeons, one anesthesiologist, one nurse and one perfusionist were trained both in drylab and wetlab settings. After this preclinical training we visited several high-volume centers with robotic mitral surgery programs. The last step for starting the program was proctored cases in our center. We determined that, the operative variables were stable after 30 cases and we may conclude that the initial learning period for such a robotic program should include 30 cases at the beginning.
There are limitations of our study. Our results are reported from a single center with a single group of operating surgeons. The number of patients is limited which makes generalizing our results difficult.
In conclusion, our early results suggest that MVR can be performed safely with the robotic technique. The main advantages of this technique over mini-thoracotomy may include a smaller thoracotomy, better visualization and dexterity.
Conclusions
Robotic MVR is feasible and safe. It can be performed with a lower mortality than expected. The robotic experience we had during the robotic mitral valve repair at the initial phase of robotic cardiac surgery programs can be transmitted in to robotic MVR operations.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sakaguchi T. Minimally invasive mitral valve surgery through a right mini-thoracotomy. Gen Thorac Cardiovasc Surg 2016;64:699-706. [Crossref] [PubMed]
- Marin Cuartas M, Javadikasgari H, Pfannmueller B, et al. Mitral valve repair: Robotic and other minimally invasive approaches. Prog Cardiovasc Dis 2017;60:394-404. [Crossref] [PubMed]
- Senay S, Gullu AU, Alhan C. Robotic mitral valve replacement for rheumatic mitral disease. Ann Cardiothorac Surg 2017;6:64-6. [Crossref] [PubMed]
- Bastopcu M, Senay S, Güllü AÜ, et al. Percutaneous cannulation for cardiopul-monary bypass in robotic mitral valve surgery with zero groin complications. J Card Surg 2022;37:280-4. [Crossref] [PubMed]
- Güllü AÜ, Şenay Ş, Ersin E, et al. Robotic-assisted cardiac surgery without aortic cross-clamping: A safe alternative approach. J Card Surg 2021;36:165-8. [Crossref] [PubMed]
- Senay S, Cacur O, Bastopcu M, et al. Robotic mitral valve operations can be safely performed in obese patients. J Card Surg 2021;36:3126-30. [Crossref] [PubMed]
- Zhao H, Gao C, Yang M, et al. Surgical effect and long-term clinical outcomes of robotic mitral valve replacement: 10-year follow-up study. J Cardiovasc Surg (Torino) 2021;62:162-8. [PubMed]
- Gao C, Yang M, Xiao C, et al. Robotically assisted mitral valve replacement. J Thorac Cardiovasc Surg 2012;143:S64-7. [Crossref] [PubMed]
- Kuo CC, Chang HH, Hsing CH, et al. Robotic mitral valve replacements with bioprosthetic valves in 52 patients: experience from a tertiary referral hospital. Eur J Cardiothorac Surg 2018;54:853-9. [Crossref] [PubMed]







