Systematic review and meta-analysis of mid-term survival, reoperation, and recurrent mitral regurgitation for robotic-assisted mitral valve repair
Introduction
Mitral regurgitation (MR) is the most prevalent form of valvular heart disease in the developed world, increasing in incidence with age, affecting 10% of patients over the age of seventy-five (1). The phenotype of valvular disease in general has changed over preceding decades. The incidence of rheumatic heart disease has fallen in industrialized countries, with degenerative valvular disease being the leading mechanism of MR (1).
The mortality associated with severe MR is 50% at five-years and up to 90% of patients will have a hospitalization for heart failure within one year (2). There is an increasing trend toward earlier intervention in asymptomatic patients (3) and is now a guideline directed therapy (4). With an ageing and more comorbid population, patients with both early and late stages of MR stand to benefit from less invasive surgical approaches.
Surgery remains the gold-standard intervention for severe mitral valve disease in operative candidates. In severe degenerative MR, mitral valve repair, where possible, is the preferred approach over mitral valve replacement (5). Robotic mitral valve surgery is an extension to the minimally invasive surgical approaches for the mitral valve. Several studies have reported the safety and efficacy with satisfactory early results of robotic mitral valve repair (RMVr) (6,7). Robotic assisted mitral valve surgery facilitates surgery through smaller incisions with improved cosmesis, resulting in faster recovery, decreased pain and shorter hospital length of stay (8). In comparison to the gold standard surgical approach, conventional sternotomy, robotic mitral valve surgery has been shown to have lower incidences of post-operative atrial fibrillation, ventilation time, intensive care unit (ICU) stay and red blood cell (RBC) transfusion (9-11).
To date, most of the literature surrounding RMVr is limited to reports with only short-term follow-up. Therefore, this systematic review sought to provide a comprehensive analysis of the available literature to determine mid-term outcomes of RMVr.
Methods
The recommendations and guidelines set forth in the updated statement by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were adhered to for the conduction of this systematic review and meta-analysis (12).
Literature search strategy
The literature search was conducted using five electronic databases including Ovid MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CCRCT), Cochrane Database of Systematic Reviews (CDSR), and Database of Abstracts of Review of Effectiveness (DARE). All five databases were searched from inception to 23rd March, 2022. The search strategy included a combination of keywords and Medical Subject Headings (MeSH) including “Robotic” OR “Robotically-assisted” OR “Robo*” AND “Mitral valve” AND “Repair” OR “Annuloplasty”. Reference lists from previous systematic reviews, meta-analyses and included articles were also reviewed to ensure no additional publications were missed.
Study selection
Study eligibility for inclusion in this systematic review and meta-analysis included those which reported mid-term outcomes for RMVr. Studies with cohorts that were either mixed without reporting separate outcomes for mitral valve repair/replacement, or different surgical approaches other than robotically assisted, were excluded. If centers/registries reported outcomes of overlapping patient series with either larger cohort size or extended follow-up, only the most complete, contemporary series was included for analysis. Abstracts, case reports, conference presentations, editorials and reviews were excluded, while included studies were limited to those in English, and only those involving human subjects. Title and abstract screening, followed by full-text review to determine included studies was performed independently by two reviewers (MLW and AE) with any discrepancies discussed until consensus reached.
Outcomes of interest
The primary outcomes of interest were mid-term (defined as five years or more) overall survival, freedom from MV reoperation and freedom from moderate or worse MR. To be eligible for inclusion, studies had to report at least one of these three primary outcomes of interest. Secondary outcomes of interest included in-hospital/thirty-day mortality, cerebrovascular accidents (CVA), reoperation for bleeding, reoperation for valve dysfunction, post-operative atrial fibrillation (POAF) and, length of ICU and hospital stay.
Data extraction
Two independent reviewers (AE and BH) extracted data directly from publication texts, tables and figures. A third reviewer (MLW) independently reviewed and confirmed all extracted data. Differences of opinions between the two main reviewers (AE and BH) were resolved through means of discussion and consensus, including the primary investigator (MLW) where necessary. Attempts were made to clarify any insufficient or indistinct data from corresponding authors of included studies where required.
Statistical analysis
Meta-analysis of proportions or means was performed for categorical and continuous variables, as appropriate, to pool the patient characteristics and aggregate operative outcomes. To facilitate this statistical pooling, the methods described by Wan and colleagues were used to calculate means and standard deviations from the median (with range or interquartile range), where reported (13). A random effects model was chosen for the statistical analyses given variability would be present in terms of differing center/surgeon experience, different repair procedures, and different operative and management protocols across the included studies. Pooled proportions are presented as N (%) with 95% confidence intervals (CI) and pooled means are presented as a mean value (95% CI). For outcome data, heterogeneity amongst studies was assessed using the I2 statistic. Thresholds for I2 values were considered as low, moderate and high heterogeneity at 0–49%, 50–74% and ≥75%, respectively (14). Meta-analysis of proportions or means were performed using Stata (version 17.0, StataCorp, Texas, USA).
Mid-term survival, freedom from mitral valve reoperation, and freedom from moderate or worse mitral regurgitation post RMVr data was calculated from aggregation of Kaplan-Meier curves from included studies, where reported, using the methods described by Guyot and colleagues (15). Aggregation of this data was performed by reconstructing individual patient data from digitized Kaplan-Meier survival curves and patient number-at-risk data. This reconstructed individual patient data was then pooled and used to generate aggregated Kaplan-Meier curves. Digitization of source Kaplan Meier curves was performed using DigitizeIt (version 2.5.9, Braunschweig, Germany) and individual patient data reconstruction analysis was performed using R (version 4.2.0, R Foundation for Statistical Computing, Vienna, Austria).
Study quality appraisal
Study quality was assessed using the modified Canadian National Institute of Health Economics (CNIHE) assessment tool for case series (16) (Table S1). Studies were considered high quality if they addressed at least seventeen of the nineteen criteria outlined in the CNIHE tool, moderate quality if twelve to sixteen criteria were addressed, and of low quality if fewer than twelve criteria were addressed. Study quality was independently assessed by two investigators (MLW and BH) with any discrepancies clarified through the means of discussion until consensus was reached.
Results
A total of 1,576 articles were identified in the electronic literature search (Figure 1). Eighty-three articles underwent full-text review after exclusion of duplicates and irrelevant studies identified through title/abstract screening. After full-text review, seventy-four articles were excluded due to not fulfilling the inclusion criteria, mainly for lacking mid-term outcome data. Moreover, several centers published multiple articles fulfilling inclusion criteria, with four studies being excluded due to overlapping cohorts (17-20). Another study which reported mid- to long-term results after endoscopic robotic mitral valve surgery in 1,257 patients by Murphy and colleagues was excluded as 7% of patients underwent mitral valve replacement (not repair) and therefore did not meet inclusion criteria. Therefore, nine studies remained after fulfilling the pre-determined inclusion criteria (21-29), with a total of 3,300 patients undergoing RMVr.
Study characteristics
Eight of the nine included studies were retrospective observational case series or cohort studies (21,22,24-29), with only one study being prospective in nature (23) (Table 1). One of the included studies was a comparative cohort study comparing RMVr to conventional sternotomy (24), therefore, only data regarding the robotic group was included in the present study. Included studies had varying patient cohort size from 110 to 1,036 patients. Pooled clinical follow-up across the included studies was 54.1 months (95% CI: 49.7–57.8 months) and pooled echocardiographic follow-up was 35.6 months (95% CI: 31.2–39.7 months). Study quality was consistent across the included studies with all nine deemed of moderate quality scoring between fourteen to sixteen points on the CNIHE assessment tool for case series (Table S2). Deficiencies in study quality tended to be due to the retrospective nature, single center study design, and poor reporting of conflicts of interest.
Table 1
| Primary Author | Year | Institution(s) | Study period | Type of study |
n | Mean clinical follow up time (months) | Mean Echocardiographic follow-up (months) | Robotic system |
|---|---|---|---|---|---|---|---|---|
| Chitwood | 2008 | East Carolina Heart Institute, East Carolina University, Greenville, North Carolina, USA | 2000–2006 | Prospective cohort | 300 | NR | 26.8±15.1 | da Vinci Surgical System |
| Yoo | 2014 | Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea | 2007–2012 | Retrospective cohort | 200 | 31.4 (12.4–42.3)* | 29.6 (14.9–45.8)* | da Vinci Surgical System |
| Kim | 2017 | University of Ulsan College of Medicine, Seoul, Republic of Korea | 2007–2015 | Retrospective cohort | 310 | 55.7 (30.3–81.3)* | NR | da Vinci Surgical System |
| Kesavuori | 2018 | Heart and Lung Center, Helsinki University Central Hospital, Helsinki, Finland. | 2011–2015 | Retrospective cohort (comparative study) | 142 | NR | 15 [3–23]* | da Vinci Surgical System Si |
| Liu | 2019 | Chinese People's Liberation Army General Hospital, Beijing, China | 2007–2014 | Retrospective cohort | 110 | 50 [1–84]** | NR | da Vinci Surgical System |
| Arghami | 2021 | Mayo Clinic, Rochester, Minnesota, USA | 2008–2019 | Retrospective cohort | 843 | 36 (13.2–72)* | NR | da Vinci Surgical System Si and Xi |
| Roach | 2021 | Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, California, USA | 2005–2020 | Retrospective cohort | 1,036 | 66 (0–180)** | 20.4 (0–180)** | NR |
| Barac | 2022 | Duke University Medical Center, Durham, North Carolina, USA | 2011 -2019 | Retrospective cohort | 133 | 38.4±32.4 | 50.4 (10.8–55.2)* | da Vinci Surgical System Si or Xi machines |
| Klepper | 2022 | Saint-Luc University Clinics, Catholic University of Louvain, Brussels, Belgium | 2012–2019 | Retrospective cohort | 226 | 39.3±26.0 | 38.1±26.5 | da Vinci Si Surgical System |
*, median and interquartile range; **, median and range. NR, not reported.
Patient baseline characteristics
Overall, the weighted pooled age of patients across the included studies was 57.5 years (95% CI: 53.2–60.7). The patient cohort across all included studies comprised of 68.6% (95% CI: 62.9–73.9) male patients. Just over one third of patients had a history of hypertension (35.3%; 95% CI: 29.5–41.4). Only a small percentage of patients had a history of prior CVA (2.9%; 95% CI: 2.2–3.6), diabetes (4.9%; 95% CI: 3.2–7.0) or chronic obstructive pulmonary disease (COPD) (3.1%; 95% CI: 1.8–4.7). Data regarding peripheral vascular disease was poorly reported across the included studies and when reported was very low (less than 1.5%). The majority of patients were in New York Heart Association (NYHA) heart failure classification I or II (79.8%; 95% CI: 67.7–89.7) and had severe MR (89.5%; 95% CI: 75.9–98.0) at the time of surgery. Most patients had myxomatous mitral valve degeneration as the aetiology of valvular disease (95.7%; 95% CI: 88.2–99.6) (Table S3) and 61.2% (95% CI: 54.9–68.6) had isolated posterior mitral valve leaflet prolapse. Other patient baseline characteristics are summarized in Table 2. Full break down of underlying mitral valve pathology can be seen in Table S1.
Table 2
| Variable | Weighted pooled estimate |
|---|---|
| Age (years), mean | 57.5 |
| Male, % | 68.6 |
| Hypertension, % | 35.3 |
| Diabetes, % | 4.9 |
| Cerebrovascular accident, % | 2.9 |
| COPD, % | 3.1 |
| Previous cardiac surgery, % | 0.5 |
| atrial arrhythmia, % | 18.1 |
| NYHA I/II, % | 79.8 |
| NYHA III/IV, % | 20.6 |
| LVEF, mean | 62.8 |
| Severe MR, % | 89.5 |
| Valve pathology—myxomatous degeneration, % | 95.7 |
| Posterior MV prolapse, % | 61.2 |
| Anterior MV prolapse, % | 14.2 |
| Bileaflet MV prolapse, % | 19.6 |
COPD, chronic obstruction pulmonary disease; NYHA, New York Heart Association heart failure classification; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MV, mitral valve.
Operative details
The operative technique for robotic access for the RMVr across the included studies varied from a 2- to 8-centimeter right incision in either the fourth or fifth intercostal space along with a varying number of other robotic access ports (Table S4). Eight of the nine included studies used the da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, California, USA) (21-27,29), with the final study not reporting the robotic surgical platform used (28). The majority of the included studies utilized femoral arterial cannulation, transthoracic aortic cross clamping and antegrade cardioplegia delivery. Pooled cross clamp and cardiopulmonary bypass (CPB) times were 75.3 minutes and 116.7 minutes, respectively. The pooled weighted rate of successful RMVr was 99.8% (95% CI: 99.4–100; I2=59%). In total, there were twenty-four conversions to sternotomy/thoracotomy across the nine included studies with a weighted pooled conversion rate of 0.6% (95% CI: 0.01–1.8; I2=87%). Majority of these conversions (fourteen) came from one study, which included the learning phase of RMVr and reported a low threshold for conversion to maximize the safety of the procedure in the learning period (24). Reasons for conversion in this study included problems with endoclamp positioning/cardioplegia delivery, suboptimal mitral valve repair, bleeding, pleural adhesions, venous return issues and robotic malfunction. Further information on the procedural details and concomitant surgical procedures can be found in Table S2.
Overall survival
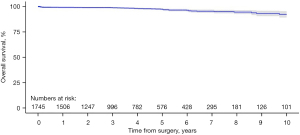
Aggregation of overall survival was performed with data from six of the included studies (21-24,26,27). Overall survival rates at one-, two-, three-, four- and five-year post RMVr were 99.2%, 99.0%, 98.9%, 98.2% and 97.4%, respectively (Figure 2). At seven-years post-operatively survival rate was 95.4% and at ten-years the overall survival rate was 92.3%.
Reoperation
Kaplan-Meier curves reporting data for freedom from MV reoperation were available in five of the included studies (22-24,26,27). Rates of freedom from MV reoperation at one-, two-, three-, four- and five-year were 97.9%, 96.2%, 95.3%, 95.0%, and 95.0%, respectively (Figure 3). At eight-year post RMVr the freedom from MV reoperation was 95.0%.
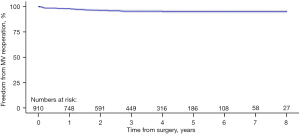
Specific data reported on long-term re-operation was available in eight of the included studies (21-28). Across these studies there were seventy-one total cases of reported MV reoperation with a weighted pooled rate of 2.2% (95% CI: 1.3–3.3; I2=61%). Data regarding time until reoperation was only available in four studies (21,23,24,28), however, the weighted pooled time to mitral valve reoperation was 23.1 months (95% CI: 19.2–29.5; I2=98.5%).
MR recurrence
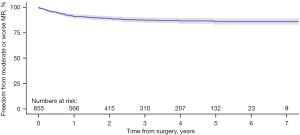
Data from Kaplan-Meier curves for freedom from moderate or worse MR was reported in four studies (22,25,26,29). Freedom from moderate or worse MR at one-, two-, three-, four- and five-year post-RMVr was 91.1%, 89.1%, 87.4%, 86.7% and 86.0% respectively (Figure 4). At seven-years post-RMVr, freedom from moderate or worse MR was 86.0%.
Secondary outcomes
All nine included studies reported early (<thirty-day) mortality rates. The weight pooled estimate of early all-cause mortality was 0.2% (95% CI: 0.04–0.4; I2=0%). Pooled rates for CVA (1.0%; 95% CI: 0.6–1.5; I2=0%) and dialysis (0.3%; 95% CI: 0.08–0.7; I2=0%) were also low. Rates of POAF were 24.2% (95% CI: 22.1–26.5; I2=0%) and reoperation for bleeding were 2.2% (95% CI: 1.1–3.5; I2=79%). Mean ICU stay was 22.4 hours (95% CI: 14.3–29.6; I2=99%) and hospital stay 5.2 days (95% CI: 4.4–6.3; I2=98%) post-RMVr. Other early post-operative outcomes are summarized in Table 3.
Table 3
| Parameter | Events/total | N | Weighted pooled estimate (%) (95% CI) | Heterogeneity I2 (%) |
|---|---|---|---|---|
| All-cause mortality | 11/3,300 | 9 | 0.2 (0.04–0.4) | 0 |
| CVA | 26/2,315 | 7 | 1.0 (0.6–1.5) | 0 |
| Dialysis | 10/1,931 | 6 | 0.3 (0.08–0.7) | 0 |
| POAF | 391/1,612 | 5 | 24.2 (22.1–26.5) | 0 |
| Superficial infection | 4/1,221 | 6 | 0.02 (0.0–0.06) | 0 |
| Reoperation bleeding | 75/3,300 | 9 | 2.2 (1.1–3.5) | 79 |
| Reoperation valve dysfunction | 14/2,725 | 6 | 0.4 (0.2–0.8) | 14 |
| ICU stay, hours | NA/1,931 | 6 | 22.4 (14.3–29.6)* | 99 |
| Hospital stay, days | NA/3,190 | 8 | 5.2 (4.4–6.3)* | 98 |
*, weighted pooled mean. N, number of studies; CI, confidence interval; CVA, cerebrovascular accident; POAF, post-operative atrial fibrillation; ICU, intensive care unit; NA, not applicable.
Discussion
RMVr has been shown to be a safe procedure with acceptable outcomes by a number of dedicated, high-volume centers worldwide (17,30,31). Robotic mitral valve surgery has also been demonstrated to have comparable short-term outcomes to the gold standard conventional sternotomy and other minimally invasive surgical approaches to the mitral valve (9-11,32). However, worldwide uptake of RMVr has been slow with the main concerns being related to the steep learning curve/operative complexity and higher associated costs (33). Several observational, single center studies have shown promising short-term outcomes, however, mid- to long-term outcomes are scarce. Therefore, the aim of this systematic review and meta-analysis was to provide a comprehensive review of all studies in the existing literature reporting mid-term outcomes after RMVr.
Supporters of robotic valve surgery promote that all primary MV disease can be repaired robotically with the advantages of reduced ICU/hospital stay, fewer RBC transfusion, improved cosmesis and improved early quality of life post-operatively (8,10). It has also been shown that more complex MV pathologies can be repaired robotically without affecting outcomes (6). In the present study, rate of successful RMVr was 99.8%, however, definitions of what defined a successful repair in the included studies was rarely reported.
One disadvantage of robotic surgery, especially cardiac surgery, is the steep learning curve associated with this surgical approach. This steep learning curve leads to longer cross clamp, CPB and operative times. In the present study, pooled cross clamp and CPB times were 75.3 and 116.7 minutes, respectively. Two of the included studies in the present study examined cross clamp and CPB times over the study period and reported significantly shorter times with greater operator experience (24,26). The study by Barac et al., which had the second smallest included cohort and a “lower volume center” had the longest reported cross clamp and CPB times at 146 and 265 minutes, respectively. When the two largest studies included in the present study (21,28) were excluded, the cross clamp and CPB times extended out to 109.9 and 165.5 minutes, respectively, which would indicate a significant reduction in operative times with greater operative and surgical team experience. Unfortunately, in the present study, only four of the included studies reported mean total operative times and these were quite heterogenous (ranging from 222 to 310 minutes).
Advantages of robotic surgery have been reported to include shorter ICU and hospital length of stay, shorter periods of ventilation, less RBC transfusion and lower rates of POAF (10). In the present study, rates of post-operative complications were low with 24.2% of patients experiencing POAF and only 1.0% experiencing CVA post RMVr. Reoperation for bleeding was low at 2.2%, which is similar to rates reported in the literature across both robotic and conventional sternotomy mitral valve surgery (32,34-36). Pooled mean ICU length of stay and hospital stay in the current study was 22.4 hours and 5.2 days, respectively. A study by Coyan and colleagues assessing 182 propensity score matched patients who underwent robotic or conventional sternotomy mitral valve surgery reported significantly longer ICU and hospital length of stay (ICU median 27.0 vs. 31.0 hours and hospital median 5 vs. 7 days, respectively) in the conventional sternotomy cohort.
Mid- to long-term survival after robotic cardiac surgery appears to be satisfactory and comparable to other surgical approaches to the mitral valve. Rates of overall survival in the present study were 97.4% at five-year and 92.3% at ten-year. These figures are comparable to those reported by Dreyfus and colleagues who reported overall survival rates of 93.6% at five-year and 86.7% at ten-year after mitral valve repair (37). Lange and colleagues who performed a propensity matched analysis of ninety-seven paired patients who underwent either right mini-thoracotomy or full sternotomy mitral valve repair reported five-year overall survival rates of 93.5% and 87.4%, respectively.
Freedom from mitral valve reoperation for RMVr also appears to be comparable to other surgical approaches. Galloway et al., reported eight-year freedom from mitral valve reoperation results of 91.0% and 95.0% for both conventional sternotomy and mini-thoracotomy mitral valve repair, respectively (38). The results from this present meta-analysis showed that at eight-years post-RMVr the rate of freedom from mitral valve reoperation was estimated to be 95.0%. Literature regarding freedom from recurrent moderate or worse MR after mitral valve repair is quite heterogenous. Rates have varied from 77.0% at five-year (39), 71.0% at seven-year (40) and 81.0% at ten-years (41). These varying differences in rates are likely due to a combination of patient selection, varying repair techniques and surgeon experience in mitral valve repair (42). In the present study, pooled rates of freedom from moderate or worse MR were comparable at 86.0% at seven-years post RMVr.
There are several important limitations to consider when interpreting the results from the present study. The observational nature of all included studies presents an inherent source of bias in the present study. Most studies also lacked clear definitions to what was deemed a successful mitral valve repair (i.e., none/trace/mild MR post repair). Another important consideration is the varying repair techniques and concomitant procedures across the included studies. Finally, significant heterogeneity was detected in the analyses of reoperation for bleeding, ICU and hospital LOS. This may reflect the limited data, differing operator experience or difference between specific unit post-operative management protocols across the included studies.
Conclusions
In summary, RMVr provides a safe and effective treatment modality for patients with MV disease with low rates of early mortality. It can be performed with low complication rates in high volume, experienced centers. RMVr can be performed with good mid-term results, including satisfactory rates of overall survival and freedom from MV reoperation. Rates of freedom from moderate or worse MR recurrence are acceptable, especially given the steep learning curve required for robotic cardiac surgery. Further high quality prospective multicenter registry data and randomized control trials are required to evaluate and compare the different surgical approaches for mitral valve repair.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Dr. SG provides consultation for Edwards Lifesciences, Johnson & Johnson, and Intuitive Surgical. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart 2016;102:75-85. [Crossref] [PubMed]
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014;63:185-6. [Crossref] [PubMed]
- Desai A, Thomas JD, Bonow RO, et al. Asymptomatic degenerative mitral regurgitation repair: Validating guidelines for early intervention. J Thorac Cardiovasc Surg 2021;161:981-994.e5. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. [Crossref] [PubMed]
- Loulmet DF, Ranganath NK, Neuburger PJ, et al. Can complex mitral valve repair be performed with robotics? An institution's experience utilizing a dedicated team approach in 500 patients†. Eur J Cardiothorac Surg 2019;56:470-8. [Crossref] [PubMed]
- Seco M, Cao C, Modi P, et al. Systematic review of robotic minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:704-16. [PubMed]
- Suri RM, Antiel RM, Burkhart HM, et al. Quality of life after early mitral valve repair using conventional and robotic approaches. Ann Thorac Surg 2012;93:761-9. [Crossref] [PubMed]
- Cao C, Wolfenden H, Liou K, et al. A meta-analysis of robotic vs. conventional mitral valve surgery. Ann Cardiothorac Surg 2015;4:305-14. [PubMed]
- Takagi H, Hari Y, Nakashima K, et al. Meta-analysis of propensity matched studies of robotic versus conventional mitral valve surgery. J Cardiol 2020;75:177-81. [Crossref] [PubMed]
- Williams ML, Hwang B, Huang L, et al. Robotic versus conventional sternotomy mitral valve surgery: a systematic review and meta-analysis. Ann Cardiothorac Surg 2022;11:490-503. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [Crossref] [PubMed]
- Institute of Health Economics, Quality Appraisal of Case Series Studies Checklist. Edmonton (AB), Institute of Health Economics, Editor. 2016.
- Maltais S, Anwer LA, Daly RC, et al. Robotic Mitral Valve Repair: Indication for Surgery Does Not Influence Early Outcomes. Mayo Clin Proc 2019;94:2263-9. [Crossref] [PubMed]
- Rodriguez E, Nifong LW, Chu MW, et al. Robotic mitral valve repair for anterior leaflet and bileaflet prolapse. Ann Thorac Surg 2008;85:438-44; discussion 444. [Crossref] [PubMed]
- Suri RM, Taggarse A, Burkhart HM, et al. Robotic Mitral Valve Repair for Simple and Complex Degenerative Disease: Midterm Clinical and Echocardiographic Quality Outcomes. Circulation 2015;132:1961-8. [Crossref] [PubMed]
- Wang Y, Gao CQ, Shen YS, et al. Echocardiographic Follow-up of Robotic Mitral Valve Repair for Mitral Regurgitation due to Degenerative Disease. Chin Med J (Engl) 2016;129:2199-203. [Crossref] [PubMed]
- Arghami A, Jahanian S, Daly RC, et al. Robotic Mitral Valve Repair: A Decade of Experience With Echocardiographic Follow-up. Ann Thorac Surg 2022;114:1587-95. [Crossref] [PubMed]
- Barac YD, Loungani RS, Sabulsky R, et al. Sustained results of robotic mitral repair in a lower volume center with extensive minimally invasive mitral repair experience. J Robot Surg 2022;16:199-206. [Crossref] [PubMed]
- Chitwood WR Jr, Rodriguez E, Chu MW, et al. Robotic mitral valve repairs in 300 patients: a single-center experience. J Thorac Cardiovasc Surg 2008;136:436-41. [Crossref] [PubMed]
- Kesävuori R, Raivio P, Jokinen JJ, et al. Early experience with robotic mitral valve repair with intra-aortic occlusion. J Thorac Cardiovasc Surg 2018;155:1463-71. [Crossref] [PubMed]
- Kim HJ, Kim JB, Jung SH, et al. Clinical outcomes of robotic mitral valve repair: a single-center experience in Korea. Ann Cardiothorac Surg 2017;6:9-16. [Crossref] [PubMed]
- Klepper M, Noirhomme P, de Kerchove L, et al. Robotic mitral valve repair: A single center experience over a 7-year period. J Card Surg 2022;37:2266-77. [Crossref] [PubMed]
- Liu G, Zhang H, Yang M, et al. Robotic mitral valve repair: 7-year surgical experience and mid-term follow-up results. J Cardiovasc Surg (Torino) 2019;60:406-12. [Crossref] [PubMed]
- Roach A, Trento A, Emerson D, et al. Durable Robotic Mitral Repair of Degenerative Primary Regurgitation With Long-Term Follow-Up. Ann Thorac Surg 2022;114:84-90. [Crossref] [PubMed]
- Yoo JS, Kim JB, Jung SH, et al. Mitral durability after robotic mitral valve repair: analysis of 200 consecutive mitral regurgitation repairs. J Thorac Cardiovasc Surg 2014;148:2773-9. [Crossref] [PubMed]
- Bush B, Nifong LW, Alwair H, et al. Robotic mitral valve surgery-current status and future directions. Ann Cardiothorac Surg 2013;2:814-7. [PubMed]
- Suri RM, Dearani JA, Mihaljevic T, et al. Mitral valve repair using robotic technology: Safe, effective, and durable. J Thorac Cardiovasc Surg 2016;151:1450-4. [Crossref] [PubMed]
- Hawkins RB, Mehaffey JH, Mullen MG, et al. A propensity matched analysis of robotic, minimally invasive, and conventional mitral valve surgery. Heart 2018;104:1970-5. [Crossref] [PubMed]
- Benmessaoud C, Kharrazi H, MacDorman KF. Facilitators and barriers to adopting robotic-assisted surgery: contextualizing the unified theory of acceptance and use of technology. PLoS One 2011;6:e16395. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
- Seo YJ, Sanaiha Y, Bailey KL, et al. Outcomes and Resource Utilization in Robotic Mitral Valve Repair: Beyond the Learning Curve. J Surg Res 2019;235:258-63. [Crossref] [PubMed]
- Dreyfus GD, Dulguerov F, Marcacci C, et al. "Respect when you can, resect when you should": A realistic approach to posterior leaflet mitral valve repair. J Thorac Cardiovasc Surg 2018;156:1856-1866.e3. [Crossref] [PubMed]
- Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4. [Crossref] [PubMed]
- Stevens LM, Basmadjian AJ, Bouchard D, et al. Late echocardiographic and clinical outcomes after mitral valve repair for degenerative disease. J Card Surg 2010;25:9-15. [Crossref] [PubMed]
- Flameng W, Herijgers P, Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation 2003;107:1609-13. [Crossref] [PubMed]
- Shimokawa T, Kasegawa H, Katayama Y, et al. Mechanisms of recurrent regurgitation after valve repair for prolapsed mitral valve disease. Ann Thorac Surg 2011;91:1433-8; discussion 1438-9. [Crossref] [PubMed]
- Kilic A, Shah AS, Conte JV, et al. Operative outcomes in mitral valve surgery: combined effect of surgeon and hospital volume in a population-based analysis. J Thorac Cardiovasc Surg 2013;146:638-46. [Crossref] [PubMed]







