Robotically assisted mitral valve surgery—experience during the restart of a robotic program in Germany
Introduction
The history of robotic surgery commenced with the first mitral valve operation performed by Alain Carpentier in 1998 using a prototype of the daVinci robotic system (Intuitive Surgical, Inc., Sunnyvale, USA) (1). Friedrich Mohr performed the first coronary anastomosis using an identical system in Leipzig, Germany, around the same time (2). Autschbach et al. (3) published the Leipzig groups’ robotic valve surgery experience as early as 2000. Thirteen of fifteen patients were operated successfully using the daVinci telemanipulation system. This initiated the uptake of robotically assisted cardiac procedures worldwide. In 2005, Nifong et al. published results of a prospective phase II Food and Drug Administration trial including ten centers from the USA with 112 patients. Despite 8% not matching the primary study endpoint of mitral regurgitation less than grade 2, no deaths or device related complications were reported (4).
In Germany, centers in Frankfurt, Munich and Dresden established robotic-assisted cardiac surgery programs and published encouraging results (5-7). Initial enthusiasm for robotic cardiac surgery, however, did not translate into routine clinical usage despite good results. The duration of a robotic procedure was significantly longer compared to minimally invasive mitral valve repair (MVR) or minimally invasive direct coronary artery bypass (MIDCAB) procedures. Costs were very high and reimbursement by the German health system was low. In addition, some of the proponents of robotic services shifted to hospitals without the administrative mindset to finance robotic surgical developments. Furthermore, the introduction of robotic surgery fell into the same phase as the introduction of transcatheter aortic valve interventions (TAVIs), for which there was likely much more support from the community of heart surgeons and cardiologists, as well as the industry, to get proper reimbursement for. By the end of the first decade of this century, no robotic cardiac operations were being performed in Germany.
Significant improvements in robotic technology as well as favorable published results from US centers (8,9) reignited interest in robotic-assisted operations in Europe with rapidly increasing operative numbers (10). Encouraged by this evolution, we started our robotic-assisted cardiac program as the first and only center in Germany in 2019. Results and safety of this initializing process are demonstrated. The focus is directed on describing the learning curve, time consumption and benefits for patients. Additionally, a cost analysis for robotic-assisted mitral surgery in the German health system was performed and compared to the alternative minimally invasive mitral valve surgery (MIMS).
Methods
Supported by a grant from the Robert Bosch Foundation, in 2018, the administration of the Robert Bosch Hospital, Stuttgart, Germany, agreed to purchase a daVinci Xi robotic system for cardiac and general surgery services. The aim was to extend the range of treatment options of minimally invasive surgery for patients. Cardiac surgery could use the DaVinci Xi for two working days per week.
The Department of Cardiac and Vascular surgery at the Robert Bosch Hospital has a long history of supporting minimally invasive cardiac surgery techniques. Greater than 75% of all cardiac operations are performed without sternotomy and/or without extracorporeal circulation. Almost all coronary operations are done by no-touch total arterial revascularization. Greater than 90% of all isolated or concomitant mitral operations are performed through a right sided mini-thoracotomy approach.
Training and establishment
Structured training of the entire surgical team was completed. For each surgeon, this included an initial visit to Dr. Husam Balkhy in Chicago who performed two live operations, forty hours of training on a simulator, completion of web-based training and examination, a training day at the IRCAD Strasbourg, with a live animal operation and a team visit to Dr. Stepan Cerny in Prague. Finally, human cadaveric training at the Orsi academy in Melle, Belgium, including a seminar on patient selection and pitfalls during robotic cardiac surgery, was completed. This culminated in the first mitral valve operation performed in Stuttgart in the presence of the proctor Dr. Stepan Cerny. Given the steep learning curve in robotic cardiac surgery, the number of surgeons (two), operative assistants (three) and nurses (four) was limited to a selected few.
From July 2019 to December 2021, 329 patients underwent robotic-assisted cardiac operations using the daVinci Xi system. In this 30-month period, 182 patients underwent robotic-assisted MVR. The results were analyzed in two groups, the first group included patients with isolated MVR with or without patent foramen ovale closure (PFO) (isolated mitral group, IMG, n=96) and the second group included patients undergoing concomitant procedures, such as tricuspid valve repair, Cox-Maze IV ablation, left atrial maze ablation, pulmonary vein isolation (PVI) and/or closure of the left atrial appendage (LAA) (complex mitral group, CMG, n=86).
Inclusion and exclusion criteria
Exclusion criteria included severe obesity and re-operations. Furthermore, patients who had evidence of severe diaphragmatic elevation, serious calcification of the mitral valve, increased probability of mitral valve replacement and ascending aneurysm diameter larger than 40 mm on computed tomography scan were excluded. In contrast, patients with pectus excavatum, and high risk patients such as those with pulmonary hypertension, increased age or high EuroSCORE II were included.
Surgical management
In addition to the standard procedures for cardiac surgery, patients received double lumen endotracheal intubation and right internal jugular vein cannulation using a 16 Fr cannula.
Cardiopulmonary bypass was established via right femoral venous drainage and returned via the right femoral artery over a 2-cm groin incision. The 1.5–2.0 cm working port was placed at the intersection of the midaxillary line and a virtual horizontal line crossing the middle of the sternum. The trocars for the daVinci arms were placed in the anterior axillary line. The port for the atrial retractor was placed lateral to the internal mammary artery, below port 2 (camera port) to avoid conflict between the robotic arms. Two sheaths were used for pericardial sutures. In cases with a high diaphragm an additional retraction suture from the membranous part of the diaphragm was used. Into the working port an Alexis wound retractor, size XXS (Applied Medical, Rancho Santa Margarita, CA, USA) was placed to secure optimal access for the surgical assistant (Figure 1).
Transthoracic clamping using the Chitwood clamp was facilitated until June 2020. Since July 2020, the IntraClude® system (Edwards Lifesciences Corp., Irvine, CA, USA) was applied for all robotic cases. Bretschneider’s solution (Custodiol®, Dr. Franz Köhler Chemie GmbH, Bensheim, Germany) was used for all patients. All patent foramen ovale were closed using polytetrafluoroethylene sutures.
CG Future® Band (Medtronic GmbH, Meerbusch, Germany) was the annuloplasty device of choice in almost all cases. Anchoring of annuloplasty devices was performed with the Cor-Knot® device (LSI solutions, Victor, NY, USA). Chordal replacement was done using the loop technique with pre-measured Chordae Loops (Santec Medical, Großostheim, Germany) or Seramon® Chordae Loops (Serag-Wiessner GmbH, Naila, Germany). Intraoperative transesophageal echocardiography for quality control of the mitral repair was completed routinely during the reperfusion period.
For concomitant cryoablation, the CryoForm® cryoablation probe (Atricure Europe B.V., Amsterdam, Netherlands) was used. Line configurations differed between Cox-Maze IV for bi-atrial ablation, left atrial maze without the right atrial lines or PVI, according to the underlying pathology.
No specific pain management was applied. All eligible patients were extubated in the operating room. According to our Enhanced Recovery After Surgery (ERAS) protocol, eligible patients were stepped down to the intermediate care (IMC) unit on the same day.
Cost analysis
The InEK GmbH (Institut für das Entgeltsystem im Krankenhaus, German Institute for Hospital Reimbursement) data set was used to compare robotic and non-robotic MVR procedures. The German Diagnosis Related Groups (G-DRG) system is the uniform case rate system for the financing of the German health care system. For the continuous evolution of this system real costs of the diagnostics and treatment of every patient were collected in representative hospitals. Participating hospitals should capture all costs for each patient. The InEK calculates the average cost in Germany. Based on this calculation, InEK determines the average administrative financial dataset for each diagnosis to inform the reimbursement for the hospitals. This InEK calculation is differentiated into different categories, e.g., personal costs, material costs, infrastructural costs, etc.
As the Robert Bosch Hospital participates in the InEK group, detailed cost data and revenues were available for every patient. The real costs of the hospital stay for each patient were compared with the hospital reimbursement. For the comparison of the balances between robotic and non-robotic surgery, all minimally invasive mitral repairs in the year 2020 were evaluated. The data during the implementation period of the program in 2019 was not representative and the data for the year 2021 is not available yet.
Statistical analysis
Data was collected prospectively in a specified database. SPSS (Version 28, IBM, USA) was used for statistical analysis. For comparison of categorical data, Chi-square test or Fisher’s exact test were used, as appropriate. Continuous data was compared using the ANOVA test for independent samples. P values below 0.05 were considered statistically significant.
Results
The mean age for all 182 mitral patients was 62.9±12.0 years. Demographic and operative data are shown in Table 1. Patients with complex mitral valve procedures (CMG) were significantly older and had an increased preoperative risk compared to patients in the IMG. Due to the multifaceted procedure in the CMG, all operative times were significantly longer.
Table 1
| Groups | Isolated mitral group (n=96) | Complex mitral group (n=86) | P value |
|---|---|---|---|
| Age (years) | 58.8±11.1 | 67.5±11.2 | <0.001 |
| Female gender (%) | 21 (21.9) | 26 (30.2) | 0.132 |
| EuroSCORE II (%) | 1.4±1.2 | 4.8±5.1 | <0.001 |
| Skin-to-skin time (min) | 220±60 | 245±64 | <0.001 |
| ECC time (min) | 166±50 | 193±57 | <0.001 |
| Cross-clamp time (min) | 88±28 | 102±31 | 0.002 |
The data are expressed as mean ± standard deviation or n (%). ECC, extracorporeal circulation.
MVR was successful in all patients. There was no patient with mitral regurgitation greater than grade I. The vast majority of them had no or trace regurgitation. Repair procedures of the mitral valve are shown in Figure 2. Patients in the CMG received more ring annuloplasty only and patients in the IMG had, in comparison, more posterior mitral valve leaflet repairs by resection maneuvers (Table 2).

Table 2
| Groups | Isolated mitral group (n=96) | Complex mitral group (n=86) | P value |
|---|---|---|---|
| Leaflet repair [%] | <0.001 | ||
| PML | 57 [60] | 36 [42] | |
| AML | 5 [5] | 8 [9] | |
| Bi-Leaflet | 31 [32] | 22 [26] | |
| Annuloplasty ring only | 3 [3] | 20 [23] | |
| Resection technique [%] | <0.001 | ||
| No resection | 43 [45] | 67 [78] | |
| Triangular resection | 18 [19] | 8 [9] | |
| Quadrangular resection | 26 [27] | 7 [8] | |
| Others | 9 [9] | 4 [5] | |
| Chordal replacement [%] | 0.685 | ||
| No chordal replacement | 36 [38] | 39 [46] | |
| PML | 28 [29] | 20 [23] | |
| AML | 8 [8] | 8 [9] | |
| Bi-Leaflet | 24 [25] | 19 [22] |
PML, posterior mitral leaflet; AML, anterior mitral leaflet.
Results for the isolated mitral repair group (n=96)
Mortality and serious complications
One patient (1.0%) died due to multi organ failure after laparotomy for abdominal bleeding. The source of hemorrhage was due to accidental liver injury by changing the right-hand instrument and dislocation of the corresponding trocar. Unfortunately, the patient suffered from silent liver cirrhosis which was not picked up preoperatively. The observed-to-expected (O/E) mortality ratio was 0.69 with an expected mortality, using the EuroSCORE II, of 1.45%±1.21%. Two patients (2.1%) suffered from a stroke, of which one had a permanent neurological deficit. Conversion to sternotomy was necessary in four cases (4.2%). The first case was a planned a priori conversion due to a Barlow’s valve requiring complex repair. In another case, the side wall of the left ventricle was perforated, likely due to the stiff probe used for the post-repair sealing check. Six patients (6.2%) required re-exploration for bleeding.
Time consumption
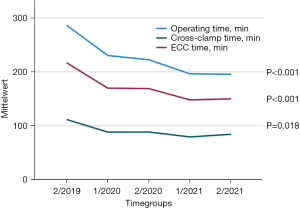
To evaluate the learning curve and time effectiveness, we compared patients grouped into six-month time periods. Whereas baseline characteristics did not differ between each time period, all operating times decreased significantly over time (Figure 3). Comparing the first with the last six month period, reduction in operating time, bypass time and cross-clamp time was 32%, 31% and 25%, respectively.
ICU and hospital stay
Median ICU stay for patients in the IMG was 22 hours (mean: 41±75 hours, range, 2–648 hours). Twelve patients (12.5%) were moved to the IMC unit on the day of surgery in accordance with the ERAS program. The median postoperative hospital length of stay was 7 days (mean: 7.6±5.3 days, range, 3–46 days).
Results for the complex mitral repair group (n=86)
Concomitant tricuspid valve repair was performed in 37 of 86 patients (43.0%). The dominant tricuspid repair technique was the annuloplasty ring implantation (n=31) with the Edwards MC3 tricuspid annuloplasty device (Edwards Lifesience Inc., Irvine, USA). In three cases, an additional Alfieri-stitch was placed. Neochord implantation was performed in one patient and resection of the infected posterior leaflet with subsequent bicuspidization was performed in two cases.
Concomitant electrical rhythm modification was performed in 63 patients (73.3%). Fifty-one patients received PVI. In twelve patients with permanent atrial fibrillation, the complete bi-atrial cryoablation (Cox-Maze IV) lesion set was performed.
Closure of the LAA was done in 63 patients (73.3%). The initial 38 patients received internal closure using a double suture line at the LAA orifice. Due to evident incomplete closure in a few cases, the LAA was resected at the base and closed by a double layer suture from inside.
Mortality and serious complications
Three patients died after the operation (3.5%). The O/E mortality ratio was 0.74 with an expected EuroSCORE II of 4.76±5.06. All three had concomitant mitral and tricuspid valve repair and Cox-Maze IV procedure (n=2) or PVI (n=1). The leading pathology for the three deceased patients was tricuspid regurgitation. Mean EuroSCORE II of these patients was 5.99%. One patient presented with right heart failure postoperatively requiring veno-arterial extracorporeal membrane oxygenation (ECMO), another patient had pulmonary failure requiring veno-venous ECMO and one patient passed away due to septic multiorgan failure following prolonged ventilation.
Cerebrovascular events occurred in two cases (2.3%) and re-exploration for bleeding was necessary in further four patients (4.6%). In three patients, liver injury with subsequent laparotomy was observed. In one case, the diaphragmatic retraction suture was the cause. In the other two cases, the mechanism causing the injury could not be explained. Conversion to sternotomy was necessary for four patients (4.6%).
Time consumption
In the CMG, the reduction of the operative times during the observation period (first versus last six months: mean operating time 263±65 vs. 233±78 minutes, mean bypass-time 222±69 vs. 187±66 minutes, cross-clamp time 133±47 vs. 100±30 minutes) remained not statistically significant.
ICU and hospital stay
ICU and hospital stay are shown in Table 3. No patients in the CMG were elected for enhanced recovery. Compared to patients in the IMG, length of both ICU and postoperative in-hospital stay was longer.
Table 3
| Groups | Isolated mitral group (n=96) | Complex mitral group (n=86) | P value |
|---|---|---|---|
| Mean ICU stay (h) | 40.7±75.5 | 60.5±105.2 | 0.072 |
| Median [range] (h) | 22 [2–648] | 23 [5–653] | – |
| Mean hospital stay (d) | 7.6±5.3 | 10.1±6.2 | 0.002 |
| Median [range] (d) | 7 [3–46] | 8 [3–33] | – |
Cost evaluation
For the evaluation of cost effectiveness, all patients undergoing robotic mitral valve surgery (RMVR) were compared to patients undergoing mini-thoracotomy mitral valve surgery (MIMS) in 2020. Patients underwent MIMS were either not eligible for RMVR (met exclusion criteria) or the robotic surgeon or the DaVinci device was not available. The actual costs were compared to the InEK calculation. Demographic data is shown in Table 4. Patients in the MIMS group were older but did not differ significantly in regards to other demographic parameters. Even bypass time and cross-clamp time were no longer in the RMVR group. The duration of length of hospital stay was 26% longer for patients in the MIMS group (12.5±4.5 vs. 9.4±3.6 days, P<0.001) compared to the RMVR group.
Table 4
| Groups | RMVR (n=57) | MIMS (n=30) | P value |
|---|---|---|---|
| Age (years) | 62±12 | 66±10 | 0.036 |
| EuroSCORE II | 2.4±2.8 | 3.0±4.0 | 0.194 |
| ECC time (min) | 170±44 | 159±34 | 0.108 |
| Cross-clamp time (min) | 91±28 | 89±19 | 0.386 |
| Hospital stay (d) | 9.4±3.6 | 12.5±4.5 | <0.001 |
The data are expressed as mean ± standard deviation. RMVR, robotic assisted mitral valve repair; MIMS, minimally invasive mitral surgery; ECC, extracorporeal circulation.
Results of cost comparison between both groups are shown in Table 5. In summary, the cost for robotic MVR was €707 higher than the InEK benchmark cost. For MIMS, the cost was €231 lower than the InEK benchmark cost. The significantly higher material costs of circa €1,500 were partially compensated by significantly lower costs for physicians and non-medical infrastructure due to the shorter hospital stay.
Table 5
| Cost groups | Real costs per case | InEK costs per case | Delta real vs. InEK costs | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| RMVR | MIMS | RMVR | MIMS | RMVR | MIMS | ||||
| Personnel costs—physicians | 3,147 | 3,387 | 4,282 | 3,936 | −1,153 | −579 | <0.001 | ||
| Personnel costs—functional services | 2,813 | 2,660 | 2,577 | 2,392 | 237 | 267 | 0.877 | ||
| Material costs | 7,732 | 5,335 | 6,348 | 5,539 | 1,384 | −204 | <0.001 | ||
| Medical infrastructure | 2,568 | 1,731 | 1,583 | 1,459 | 985 | 273 | <0.001 | ||
| Non-medical infrastructure | 2,699 | 3,147 | 3,484 | 3,165 | −764 | −18 | <0.001 | ||
| Mean costs | 18,960 | 16,259 | 18,253 | 16,490 | 707 | −231 | 0.215 | ||
RMVR, robotic mitral valve repair; MIMS, mini-thoracotomy mitral valve surgery.
Discussion
Despite the evolution of robotic cardiac surgery in the United States and other countries, in Germany, this innovative minimally invasive cardiac surgical technique was discontinued circa 15 years ago. The most important arguments against robotic cardiac surgery in Germany apply to the very high cost of acquisition and maintenance as well as the high operative time consumption. Encouraged by the available evidence for advantages and safety of robotic procedures, the aim was to establish robotic cardiac surgery in our department for further reduction of surgical trauma (11,12). In our opinion, a well-established minimally invasive cardiac program is one of the most important prerequisites for the successful integration of robotic cardiac surgery into the clinical routine.
Following intensive preparation, the establishment phase was relatively short without increased risks for the patients. The prolonged operative times at the beginning of the program was reduced significantly by focusing on structured and standardized operation processes. As early as 2020, fewer than one year after starting the program, operation times were no different to conventional minimally invasive mitral repair operations.
One advantage of robotic assistance is the precise handling inside the heart and inside the left ventricle, which is visually easier and clearer compared to MIMS. Consequently, complex leaflet repair was performed more frequently. Particularly, maneuvers at the anterior leaflet and bi-leaflet chordal replacement were applied more frequently compared to MIMS in our department. For example, we had three patients with mitral endocarditis who received multiple repair maneuvers, such as leaflet resection, pericardial patch insertion into the anterior mitral leaflet and double triangular resection (1×) or chordal replacement to the posterior mitral leaflet (2×) in the same patient. Better ergonomics for the surgeon is another big advantage.
Mortality was low compared to conventional minimally invasive mitral repair surgery. The mortality rates of 1.0% for patients with isolated mitral repair and of 3.5% for patients with complex mitral repair were comparable to the results of the 2020 European survey, which included almost all European centers (13). Other data describe mortality rates for the isolated MVR between 0.1% and 0.8% (11,12). However, comparison is difficult due to the difference in the inclusion and exclusion criteria. For patients with complex mitral repair and combined mitral and tricuspid valve repair, the comparison of mortality rates is much more problematic due to the high variability of procedural risks. Mortality rates up to 9.6% have been reported (13). For both of our groups, the isolated as well as complex mitral repair group, the O/E mortality ratio was lower than 1. The one death in the isolated mitral repair group was associated with serious liver injury in a patient with liver cirrhosis. Liver injury was observed in four patients. This complication was not observed in MIMS patients and has not been reported elsewhere. The occurrence of this complication was not dependent on experience. Three of these four events occurred in the last year. Consequently, preventative measures were specified including: placement of the caudal trocar as cranial as possible and as far away as possible from the diaphragm, every change of instruments has to be performed under direct endoscopic vision, and patients with high diaphragm were excluded from a robotic approach. For patients with apparent right heart insufficiency, robotic repair would be indicated more restrictively, due to the significant higher mortality risk. In contrast to recommendations by Gillinov et al., patients with severe pectus excavatum were not excluded from our robotic assisted MVR series (11). The three patients with a complete left sided heart were not eligible for conventional sternotomy. In these cases, robotic assistance has the advantage that the distance between chest wall and mitral valve is not a limiting factor as seen in MIMS.
A big advantage of robotic assistance is the avoidance of pain for the patient. No additional pain management was required in our experience. From our observation, most patients were almost pain-free from the third postoperative day. This significant pain reduction compared to “conventional” MIMS led to faster mobilization. Thus, discharge is possible earlier than for MIMS patients. In-hospital stay was reduced by 25%. This reduction in in-hospital stay resulted in lower costs for the hospital treatment. Coyan et al. demonstrated no extra costs for robotic mitral valve surgery (14). Because there is no re-imbursement for robotic devices in Germany, the overall cost per operation is circa €900 (5%) higher compared to MIMS.
In summary, the re-establishment of a robotic cardiac surgery in Germany was successful. The success was based on a good baseline volume of mitral operations, excellent experience with mitral repair through mini-thoracotomy, and focused preparation. This phase included a stepwise approach with simulation and mock operations with the whole team. More complex repairs were achieved with robotic assistance as compared to the classical MIMS approach. The whole spectrum of MVRs including concomitant procedures such as tricuspid valve repair, atrial cryoablation and LAA resection could be implemented with acceptable mortality and morbidity. O/E mortality rates were lower than predicted by EuroSCORE II. The avoidance of liver injury observed only during robotic assisted mitral repair is one focus for the future. The robotic assistance allows significant improvements to postoperative recovery with significant shortening of the hospital stay. Hospital stay for robotic assisted MVR is shorter compared to MIMS. In the German health system, overall costs are still higher for the robotic assisted procedures than for MIMS, but only by about 5%.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [Crossref] [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced coronary artery bypass surgery. J Thorac Cardiovasc Surg 1999;117:1212-4. [Crossref] [PubMed]
- Autschbach R, Onnasch JF, Falk V, et al. The Leipzig experience with robotic valve surgery. J Card Surg 2000;15:82-7. [Crossref] [PubMed]
- Nifong LW, Chitwood WR, Pappas PS, et al. Robotic mitral valve surgery: a United States multicenter trial. J Thorac Cardiovasc Surg 2005;129:1395-404. [Crossref] [PubMed]
- Dogan S, Aybek T, Andressen E, et al. Totally endoscopic coronary artery bypass grafting on cardiopulmonary bypass with robotically enhanced telemanipulation: report of forty-five cases. J Thorac Cardiovasc Surg 2002;123:1125-31. [Crossref] [PubMed]
- Kappert U, Cichon R, Schneider J, et al. Closed-chest coronary artery surgery on the beating heart with the use of a robotic system. J Thorac Cardiovasc Surg 2000;120:809-11. [Crossref] [PubMed]
- Bauernschmitt R, Feuerstein M, Traub J, et al. Optimal port placement and enhanced guidance in robotically assisted cardiac surgery. Surg Endosc 2007;21:684-7. [Crossref] [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e804. [Crossref] [PubMed]
- Pettinari M, Navarra E, Noirhomme P, et al. The state of robotic cardiac surgery in Europe. Ann Cardiothorac Surg 2017;6:1-8. [Crossref] [PubMed]
- Nifong LW, Rodriguez E, Chitwood WR Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [Crossref] [PubMed]
- Gillinov AM, Mihaljevic T, Javadikasgari H, et al. Early results of robotically assisted mitral valve surgery: Analysis of the first 1000 cases. J Thorac Cardiovasc Surg 2018;155:82-91.e2. [Crossref] [PubMed]
- Chitwood WR Jr. Robotic mitral valve surgery: overview, methodology, results, and perspective. Ann Cardiothorac Surg 2016;5:544-55. [Crossref] [PubMed]
- Cerny S, Oosterlinck W, Onan B, et al. Robotic Cardiac Surgery in Europe: Status 2020. Front Cardiovasc Med 2021;8:827515. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]







