Robotic mitral repair: maintaining quality at all levels of complexity
Introduction
Since the early experiences of robotic mitral valve surgery promulgated by Carpentier (1), Chitwood (2) and Murphy (3), global adoption has been slow and generally limited to less complex repairs in lower surgical risk patients. Our Cleveland Clinic colleagues reported the safety and effectiveness of robotic mitral valve surgery for focal degenerative disease in their first 1,000 cases (4). They proposed a conservative screening algorithm that excluded mitral annular calcification (MAC), pulmonary hypertension and several anatomic complexities. Over the last decade, however, advances in robotic technology and perfusion strategies, as well as increased surgical experience with excellent outcomes have enabled many experienced robotic surgeons to apply nearly all standard repair techniques to a broader range of pathology (5-7). Nevertheless, there have been only a few systematic reports of a robotic approach in complex mitral valve disease or higher risk groups (8-12), indicating a potential gap in practice among the robotic cardiac surgeons, as well as a gap in achieving the full potential of robotic technology in managing more challenging patients.
This review summarizes our overall approach, extended indications and technical aspects of robotic complex mitral valve repair.
Complex mitral valve repair: are we ready?
Successful robotic cardiac surgery involves a team that includes surgeons, anesthesiologists, nurses, operating room technologists and perfusionists. An experienced team is particularly important when one starts to advance through pathoanatomic complexity. The surgeon must be fluent in applying multiple leaflet repair techniques, different types of annuloplasty rings, as well as strategies to address broader pathologies.
Safety remains the paramount consideration in approaching robotic mitral valve surgery. Following this tenet, the Cleveland Clinic group suggested a conservative protocol that excludes reoperations, MAC, pulmonary hypertension, any aortic regurgitation, left ventricular dysfunction and several anatomic features (4). We very much agree with these important principles when a team commences robotic mitral surgery. However, we believe that once the learning curve has been crested and experience gained, most, if not all, of these elements may be safely navigated. To examine our relative “all comers” strategy to robotic mitral valve surgery, we performed a propensity-matched analysis of robotic versus sternotomy mitral valve repair or replacement (13). Both groups received the same repair techniques and there was no preselection based on pathoanatomic complexity or bi-leaflet disease. We demonstrated not only that robotic mitral surgery is reproducible, durable and standardized in all patients with degenerative disease, but also that the overall cost at one year is neutral between the two approaches. Similarly, a multi-institutional study of outcomes in matched elderly groups highlighted comparable outcomes between robotic and sternotomy mitral valve repair (14).
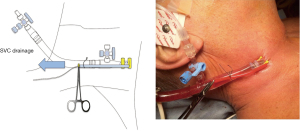
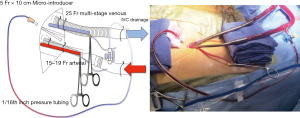
Our approach to cardiopulmonary bypass for robotic mitral valve repair includes bicaval cannulation for venous drainage, commencing with percutaneous cannulation of the superior vena cava via the jugular vein, as depicted in Figure 1. To maximize safety and reproducibility, we prefer performing femoral arterial and venous cannulation directly through a 1-cm skin incision using the Seldinger technique and transesophageal echocardiographic guidance, and placing a distal perfusion catheter in all patients, as shown in Figure 2. Our standard platform is a minimally invasive 3-cm lateral thoracotomy incision at the 4th intercostal space at the level of the anterior axillary line, followed by additional port placement (Figure 3). This identical platform may be utilized for all complex mitral repairs and may also be used for multiple other concomitant procedures such as tricuspid valve repair or replacement, biatrial cryothermic Cox Maze and robotic aortic valve replacement (15). For all robotic mitral repair surgeons, comfort with resection and non-resection techniques is recommended to enable seamless adaptation to varied pathologies with a lesion-specific strategy as complexity increases (16).
Respectful resection
There are two broad approaches to simple mitral valve repair—segmental resection with leaflet reconstruction or implantation of polytetrafluoroethylene (PTFE) neochords without resection, leading to the relative “resect vs. respect” debate. We present a case of mitral valve repair using a technique we call “respectful resection” that incorporates elements of both approaches. In a patient with posterior leaflet prolapse without a flail segment and with significant predictors of systolic anterior motion (SAM) after repair, a triangular resection is performed first (17). Next, a simple PTFE chord is placed first through the base of the papillary muscle as a simple, non-pledgetted stitch. One limb is passed through the lateral aspect of the leaflet and the other limb through the medial aspect. A single tie is placed to establish the chordal length, followed by running each suture limb to close the triangular defect in two layers. Once completed, the suture is tied at the base of the annulus, followed by placement of a flexible band to normalize the relationship of the anterior and posterior leaflets.
Sliding valvuloplasty
While reduction of posterior leaflet height may be accomplished without resection using artificial chords, a sliding valvuloplasty may be required in order to avoid SAM (18). In the subset of patients with an acute aortomitral angle and bi-leaflet disease, including those with forme fruste disease and diffuse myxomatous degeneration, we perform a debulking procedure that is commenced by P2 triangular resection. To decrease posterior leaflet height, we first undermine and excise a small wedge from the base of each side of the resection. We then detach the leaflet from the annulus nearly to each trigone. Next, a sliding valvuloplasty is performed in a stepwise manner using multiple figure-of-eight, 4-0 monofilament sutures (Cardionyl, Peters Surgical, Plymouth, MA). Once sliding has been completed, a completion valvuloplasty is performed using simple or figure-of-eight interrupted 4-0 monofilament sutures. A semirigid band is then implanted using interrupted sutures to stabilize the annulus and support leaflet coaptation.
Anterior leaflet disease
Some surgeons consider anterior leaflet pathology to be of augmented complexity and not manageable robotically. We argue that significant anterior leaflet flail and substantial mitral valve insufficiency is more amenable to a robotic approach than an open technique because the robotic approach affords significantly enhanced visualization of the subvalvular apparatus, particularly the secondary strut chords. Using the flexibility and magnification of the robot’s camera and its precise instrumentation, the secondary and tertiary fan chordae are clearly identified and divided. These chordae tendineae may then be selectively transferred in an ipsilateral fashion to the leading edge of the leaflet to create a relatively simple solution for an otherwise potentially complex problem. Occasionally, a circumferential rigid ring is helpful to augment the coaptation depth and avoid SAM.
Acute endocarditis
Active acute endocarditis has been another relative contraindication to the robotic approach, though leaflet patch repair, simple hole closure and artificial chord placement have been reported by other groups (19). In the attached video, we illustrate the approach we have used on multiple occasions to repair complex endocarditis. A 22-year-old woman presented with endocarditis from intravenous drug abuse and complicated mitral pathology, including an annular abscess at the level of the right trigone. Standard principles for management of a surgical infection were followed, beginning with debridement of all devitalized and infected tissue. The resulting annular and leaflet defect can be repaired with autologous pericardium using a “bridging gap” technique to reconstruct the trigone (18). This technique results in reconstruction of the commissure and trigone. An annuloplasty is performed at the end to complete the repair.
Mitral annular calcification
MAC can be a vexing problem, once considered a contraindication to the robotic approach (20,21), but this too can be handled robotically with experience. In contrast to the en bloc resection technique described by Loulmet et al. (22), we perform a more selective and less radical resection of MAC given the variations in depth and degree of posterior mitral leaflet impingement (23). We illustrate an example of MAC debridement and use of the anterior leaflet as an annular patch to facilitate mitral valve replacement in an elderly woman who had initially been turned down for a transcatheter mitral valve replacement due to a narrow left ventricular outflow tract. With excellent bedside assistance and utilizing both resection and ultrasonic aspiration methods, the MAC was navigated robotically. When planning for replacement, we utilize the anterior leaflet as an onlay patch placed with interrupted 4-0 monofilament sutures. The mitral valve replacement was performed using 2-0 braided, non-pledgetted sutures in standard robotic fashion.
Approaching complex mitral valve disease
In summary, our position is that frail or elderly patients with multiple comorbidities and/or complex mitral valve pathology should not be denied robotic surgery if they have anatomy appropriate for peripheral cannulation and perfusion. In fact, these higher risk or frail patients with complex pathologies may be the ones who stand to benefit most from a robotic-assisted non-sternotomy approach to complex pathology.
A successful complex robotic repair program involves the following four pillars (Figure 4):
- Mastery of complex mitral repair in sternotomy cases;
- Experience with minimally invasive right thoracotomy and peripheral cardiopulmonary bypass;
- Excellence in preoperative and intraoperative imaging including high quality transesophageal echocardiography and computed tomography (CT) reconstruction to provide essential pathoanatomic-directed mitral planning prior and during the operation;
- Team excellence (including anesthesia, nursing and perfusion support).
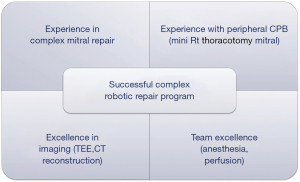
All four elements are essential as one approaches more advanced pathologies and patient complexity.
Conclusions
Robotic mitral valve surgery has evolved over the past decade with advancement in technology, technique and team experience. The associated improved outcomes with advanced pathologies and patients have led to more providers and patients seeking access to robotic cardiac surgery. Successful programs require well-trained teams following a stepwise progression to incorporate advancing pathologies while always remaining exceptionally dedicated to maintaining quality at all levels of complexity.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carpentier A, Loulmet D, Aupecle B, et al. Computer-assisted cardiac surgery. Lancet 1999;353:379-80. [Crossref] [PubMed]
- Rodríguez E, Kypson AP, Moten SC, et al. Robotic mitral surgery at East Carolina University: a 6 year experience. Int J Med Robot 2006;2:211-5. [Crossref] [PubMed]
- Murphy DA, Miller JS, Langford DA, et al. Endoscopic robotic mitral valve surgery. J Thorac Cardiovasc Surg 2006;132:776-81. [Crossref] [PubMed]
- Gillinov AM, Mihaljevic T, Javadikasgari H, et al. Early results of robotically assisted mitral valve surgery: Analysis of the first 1000 cases. J Thorac Cardiovasc Surg 2018;155:82-91.e2. [Crossref] [PubMed]
- Murphy DA, Moss E, Binongo J, et al. The Expanding Role of Endoscopic Robotics in Mitral Valve Surgery: 1,257 Consecutive Procedures. Ann Thorac Surg 2015;100:1675-81; discussion 1681-2. [Crossref] [PubMed]
- Suri RM, Burkhart HM, Daly RC, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc Surg 2011;142:970-9. [Crossref] [PubMed]
- Ramzy D, Trento A, Cheng W, et al. Three hundred robotic-assisted mitral valve repairs: the Cedars-Sinai experience. J Thorac Cardiovasc Surg 2014;147:228-35. [Crossref] [PubMed]
- Suri RM, Taggarse A, Burkhart HM, et al. Robotic Mitral Valve Repair for Simple and Complex Degenerative Disease: Midterm Clinical and Echocardiographic Quality Outcomes. Circulation 2015;132:1961-8. [Crossref] [PubMed]
- Loulmet DF, Ranganath NK, Neuburger PJ, et al. Can complex mitral valve repair be performed with robotics? An institution's experience utilizing a dedicated team approach in 500 patients†. Eur J Cardiothorac Surg 2019;56:470-8. [Crossref] [PubMed]
- Murphy DA, Jonsson AA, Halkos ME. Endoscopic Robotic Mitral Valve Surgery in Patients With Previous Sternotomy Cardiac Surgery. Innovations (Phila) 2022;17:297-303. [Crossref] [PubMed]
- Tang RC, Murphy DA, Moss E. Choosing the Ideal Candidate for a Robotic Valve Intervention. Can J Cardiol 2021;37:1117-20. [Crossref] [PubMed]
- Ranganath NK, Loulmet DF, Neragi-Miandoab S, et al. Robotic Approach to Mitral Valve Surgery in Septo-Octogenarians. Semin Thorac Cardiovasc Surg 2020;32:712-7. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]
- Wang A, Brennan JM, Zhang S, et al. Robotic Mitral Valve Repair in Older Individuals: An Analysis of The Society of Thoracic Surgeons Database. Ann Thorac Surg 2018;106:1388-93. [Crossref] [PubMed]
- Badhwar V, Wei LM, Cook CC, et al. Robotic aortic valve replacement. J Thorac Cardiovasc Surg 2021;161:1753-9. [Crossref] [PubMed]
- Alreshidan M, Herron RD, Wei LM, et al. Surgical Techniques for Mitral Valve Repair: A Pathoanatomic Grading System. Semin Cardiothorac Vasc Anesth 2019;23:20-5. [Crossref] [PubMed]
- Roberts HG, Rankin JS, Wei LM, et al. Respectful resection to enhance the armamentarium of mitral valve repair: Is less really more? J Thorac Cardiovasc Surg 2018;156:1854-5. [Crossref] [PubMed]
- Murashita T, Raffa G, Wei L, et al. Robotic mitral repair with sliding leaflet valvuloplasty and remodelling partial annuloplasty. Multimed Man Cardiothorac Surg 2016;2016:mmw013. [Crossref] [PubMed]
- Chi NH, Huang CH, Huang SC, et al. Robotic mitral valve repair in infective endocarditis. J Thorac Dis 2014;6:56-60. [PubMed]
- Gillinov AM, Suri R, Mick S, et al. Robotic mitral valve surgery: current limitations and future directions. Ann Cardiothorac Surg 2016;5:573-6. [Crossref] [PubMed]
- Asil S, Murat E, Barış VÖ, et al. Caseous calcification of the mitral annulus; scary image during robotic surgery. J Card Surg 2020;35:1145-7. [Crossref] [PubMed]
- Loulmet DF, Ranganath NK, Neragi-Miandoab S, et al. Advanced experience allows robotic mitral valve repair in the presence of extensive mitral annular calcification. J Thorac Cardiovasc Surg 2019; Epub ahead of print. [Crossref] [PubMed]
- Badhwar V. Commentary: Robotic approach to mitral annular calcification-Are we doing more with less, or is less still more? J Thorac Cardiovasc Surg 2020; Epub ahead of print. [Crossref] [PubMed]







