Robotic mitral valve repair in National Taiwan University Hospital: 10-year results
Introduction
Minimally invasive surgery in mitral valve disease is ultimately done to increase the benefit to the patient, principally, to reduce surgical trauma and obtain better post-operative results. Totally endoscopic, robotic-assisted approaches have become increasingly popular for surgeons in treating mitral valve disease. Surgeons tend to use the da Vinci system (Intuitive Surgical, CA, USA) completely endoscopically, as the console is remote from the patient. The 3-dimensional, high-resolution imaging provided by the robotic endoscope not only magnifies the surgical field, but also extends the operator’s vision around the submitral apparatus (1-3). The robotic arm system with functional wrist inputs and tremor-steady motion mitigation makes the surgery far more accurate and precise. With the help of robotic inputs, the patients can maintain thoracic cage integrity, reduce surgical trauma, and return to normal functional activity faster (1-5). We describe the National Taiwan University Hospital’s 10-year experience with totally endoscopic, robotic-assisted mitral valve repair procedures for severe mitral regurgitation.
Methods
We performed a retrospective observational cohort study of patients undergoing totally endoscopic, robotic-assisted isolated or concomitant mitral valve repair for severe mitral regurgitation at National Taiwan University Hospital. Between January 2012 and September 2022, 450 consecutive patients underwent robotic mitral valve repair, among them 272 (60.4%) were isolated mitral repair and combined additional (one or more) cardiac procedures were performed in 178 (39.6%) patients. Additional procedures included: ablation for atrial fibrillation, left atrial appendage closure, tricuspid valve repair, patent foramen ovale closure, atrial septal defect closure, left atrial thrombectomy, septal myectomy for hypertrophic obstructive cardiomyopathy and aortic valve replacement. There were 166 (36.9%) patients with atrial fibrillation pre-operatively and combined ablation surgery was performed in 136 (81.9%) of them. All procedures were completed by a single surgical team with da Vinci Si and Xi Robotic Systems. We excluded patients undergoing mitral valve replacement due to rheumatic valve disease, heavily calcified leaflets, and those who decided to those who decided to have their valve replaced preoperatively.
Surgical techniques
All patients were screened with computed tomography scans for peripheral bypass feasibility. If there was strong evidence of iliac artery calcification, stenosis, and/or a porcelain aorta, then the patient would be excluded from minimally invasive surgery. In a patient older than 55 years, or one with one with calcification of their coronary arteries on computed tomography, then coronary artery angiography will be performed (6,7). Peripheral cannulation was mostly performed using the right groin. Our setup for peripheral cannulation has been described previously. In half of the patients, the right common femoral artery was cannulated percutaneously under ultrasound guidance. Two Proglide suture systems were used for the arterial cannulation. In the other group of patients, the femoral artery was exposed with a 2 cm transverse incision over the groin and direct cannulation completed. For venous cannulation, the right jugular and right femoral veins were utilized. A 15-Fr venous cannula was introduced in the jugular vein; 21–25 Fr venous cannulae were used for femoral vein cannulation, with all procedures monitored under ultrasound and transesophageal echocardiography guidance (7-9).
Our operative set-up has been described previously. In brief, we used single lumen endotracheal intubation for airway security, and following the anesthetic preparation, a 3 cm mini-thoracotomy, at the right 4th intercostal space was performed. We used soft tissue protectors to expose the port without adopting any rib retractors. Usually, the 3rd and 6th intercostal spaces were used for the insertion of left and right working ports respectively (Figure 1A,1B). After cardiopulmonary bypass (CPB) is initiated, the pericardium was opened and we used a long shaft cardioplegic needle on the aortic root and the detachable Glauber clamp was inserted through the working port into the thoracic cage (10).

Mitral valve repair techniques
All the patients selected with severe, symptomatic mitral regurgitation underwent transthoracic echocardiography and intra-operative transesophageal echocardiography to determine the lesion site. The repair techniques were dependent on the lesion location, and different repair strategies were selected using our repair algorithm (Figures 2,3). We standardized the preparing and robotic platform steps, along with mitral repair strategies to ensure stable outcomes.

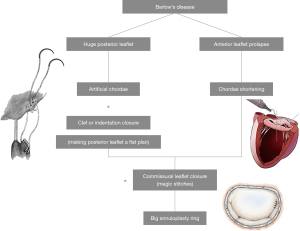
Posterior leaflet (Figure 2)
When the lesion is on the posterior leaflet, most of the time, we use artificial chords to secure the prolapsed leaflet and the papillary muscle using 4-O Gore-Tex sutures. The length of the artificial chord is determined by the annulus height and is adjusted during a saline test. Any indentation or clefts are closed to ensure a smooth coaptation zone and provide long-term durability.
Commissural leaflet (Figure 2)
If the lesion is around the commissural area, the commissure will be closed by edge-to-edge stiches between nearby anterior and posterior leaflets, or with a magic stitch. If the lesion involves the annulus around the commissure, a patch repair will be adopted to close the commissure and maintain the annulus morphology.
Anterior leaflet (Figure 2)
In the anterior leaflet, there are three scenarios. First, anterior leaflet prolapse where healthy and strong secondary chords exist—in this condition we will perform chordae transfer. Second, anterior leaflet chordae rupture or prolapse without healthy secondary chords—here we will use artificial chords on the anterior leaflet. The adjustment of the chord length on the anterior leaflet is dependent on the annulus height and is adjusted during a saline test. Third, if the anterior leaflet has mild prolapse, sometimes called pseudo-prolapse, then we will be using a chordae shortening method to shorten the chordae by 2–3 mm on the leaflet side.
Barlow’s disease (Figure 3)
Barlow’s disease requires a combination of the techniques from every lesion site (Figure 2). The techniques applied are listed on Figure 2.
Annuloplasty ring
All of the degenerative and secondary mitral regurgitation cases will require the annulus to be secured by whole ring annuloplasty. We use standard interrupted braded polyester sutures on the annulus. All the degenerative mitral repair cases are completed using true-sized annuloplasty rings, and the secondary mitral valves are completed using down-sized rings. The ring is secured either using knot pusher or knot tightening devices.
Robotic instruments
On the robotic right arm, we use monopolar curved scissors for cutting and coagulation, a large SutureCut needle driver when suturing with cutting functionality that permits surgeons to cut the suture whenever necessary without changing instruments. On the left arm, we routinely use DeBakey forceps. The atrial retractor we use is a short right-sided atrial retractor to elevate the left atrium and explore the mitral valve.
Data collection & statistical analysis
Perioperative variables, demographics and early clinical outcomes were prospectively recorded. Categorical parameters are presented as counts and percentages, whilst continuous parameters are presented as mean ± standard deviation.
Results
The Euroscore II estimate mortality was 3.1%±2.7%. The mitral valve lesion was located in the posterior leaflet in 167 patients, anterior in 92, combined in 70, commissural in 69, and Barlow’s disease in 41 (Table 1).
Table 1
| Variables | N=450 |
|---|---|
| Age (years) | 56.8±17.5 |
| Male gender | 279 (62.0%) |
| NYHA class I−II | 291 (64.7%) |
| NYHA class III−IV | 159 (35.3%) |
| BSA | 1.87±0.22 |
| Diabetes mellitus | 126 (28.0%) |
| Hypertension | 135 (30.0%) |
| Chronic obstructive pulmonary disease | 32 (7.1%) |
| Peripheral artery disease | 0 (0%) |
| Cerebrovascular disease | 8 (1.7%) |
| Atrial fibrillation | 166 (36.9%) |
| LVEF ≤50% | 12 (10.3%) |
| EuroScore II | 3.1±2.7 |
| Cardiac procedure | |
| Isolated valve repair | 272 (60.4%) |
| Valve repair + combined AF ablation | 136 (30.2%) |
| Mitral combine tricuspid repair | 52 (11.5%) |
| Mitral combine ASD or PFO closure | 12 (2.7%) |
| Mitral repair combines aortic valve replacement | 5 (1.1%) |
| Mitral valve lesion location | |
| Posterior | 167 (37.1%) |
| Anterior | 92 (20.4%) |
| Combine | 70 (15.6%) |
| Commissure | 69 (15.3) |
| Barlow’s disease | 41 (9.1%) |
| Secondary | 11 (2.4%) |
Data are expressed as mean ± standard deviation or number (%). NYHA, New York Heart Association; BSA, body surface area; LVEF, left ventricular ejection fraction; AF, atrial fibrillation; ASD, atrial septal defect; PFO, patent foramen ovale.
Learning curve
The robotic learning curve was demonstrated by the operation time and CPB time in the first consecutive 30 isolated mitral valve repair cases (Figure 4). Initially the operation time, bypass time and console time were more than 350 minutes, but within 10 cases, the procedure time can be reduced a reasonable amount. The average CPB time was 124±42 minutes, and the average operation time was 165±51 minutes. Mean intensive care unit stay time was 26.5±26.0 hours. Postoperative stroke was observed in one (0.22%) patient and new-onset atrial fibrillation was observed in 71 (15.78%) patients. Perioperative and 30-day mortality was observed in one (0.22%) patient. All patients were in less than moderate mitral regurgitation and 422 (93.78%) had none or trace regurgitation at discharge. Postoperative clinical outcome data are presented in Table 2. Freedom from moderate mitral regurgitation was 97.6% at 10 years (Figure 5), and freedom from reoperation for the mitral valve was 98%; 9 patients required a re-do mitral valve surgery, 3 because of severe mitral regurgitation reappearance, 4 because of symptomatic hemolytic anemia due to mitral regurgitation and 2 because of repeated infective endocarditis.
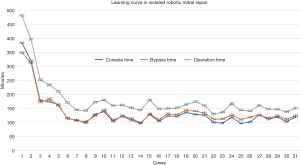
Table 2
| Outcomes | N=450 |
|---|---|
| Conversion | 0 (0%) |
| Reoperation check bleeding | 2 (0.44%) |
| Mechanical ventilation time (h) | 6.5±18.4 |
| ICU stay time (h) | 19±28.0 |
| Post-operative renal failure | 0 (0%) |
| New onset atrial fibrillation | 71 (15.78%) |
| Postoperative stroke | 1 (0.22%) |
| Postoperative dialysis | 0 (0%) |
| Reintubation | 2 (0.44%) |
| 30-day mortality | 1 (0.22%) |
| Hospital stay (d) | 7±2.9 |
| Mitral valve condition at discharge | |
| None-trace | 422 (93.78%) |
| Mild | 28 (6.22%) |
| Moderate | 0 (0%) |
| LVEF (%) | 52.3±14.7 |
Data are expressed as mean ± standard deviation or number (%). ICU, intensive care unit. LVEF, left ventricular ejection fraction.
Discussion
In this study, we have explored the outcomes of 450 mitral valve repairs from 2012 to 2022, a program set up and operated by a single experience team. The learning curve can be shortened after establishing a standardized robotic protocol (Figure 4). With the standardized protocol, all the team members know what to do at every step, allowing for the operation and CPB time to be well-controlled within 10 isolated mitral valve repair cases. Our average operation time for mitral repair was 165±51 minutes, which is reasonable in mitral valve surgery (5,11).
As experience grows with robotic techniques and more cardiac surgeons become proficient with this innovative technology, the volume of robotic cardiac procedures around the world will increase, no doubt helped along by the introduction of new robotic systems and patient demand (12-14). In this single team, pre-operative intention to repair the mitral valve is determined by valve pathology. In those with degenerative valve disease and treatable secondary mitral regurgitation patients, after shared decision making with patients, the repair rate was 100%. We excluded those with rheumatic mitral disease and calcified leaflets with mitral stenosis. In advanced, experienced teams like Loulmet, the mitral repair boundaries can be extended in severe calcification, but this is not routine in our center (11,15). We also excluded infected endocarditis with destroyed leaflets of more than half of the portion of the posterior leaflet, or more than 1/3 of anterior leaflet. Those patients received robotic mitral valve replacements.
Patient selection
Patient selection is very important, but never more so than in the beginning of a robotic program. We do not use peripheral bypass in patients with iliac artery stenosis, or those with dense pleural adhesions. Those with unsuitable peripheral bypass conditions, severe mitral annular calcification, more than moderate aortic regurgitation, poor ventricular function and high pulmonary artery pressures, will not be selected in the early phase of a program. After 1–2 years of maturation of the team, we will operate under more complex conditions. Our cohort had very good short-term post-operative outcomes. With proper screening and selection of patients, short-term surgical outcomes can be comparable to, or even better than sternotomy (13,16).
Perioperative outcomes
Experienced groups have now reported on thousands of patients collectively who have undergone robotic-assisted mitral repair, with a hospital mortality rate of less than 0.9%, stroke rate of 0.6% to 1.7%, re-exploration for bleeding of 2.2% to 4.7%, and rare chest wall infections (5,11,15,17). In our experience, the conversion rate was zero, and stroke and mortality rates of 0.2%. These intraoperative results stem from several key efforts.
First, adequate patient screening. We don’t do robotic approaches on high-risk patients in the beginning stages of a robotic program until the whole team becomes familiar with all operative steps. Second, make standardized procedures for every step in our robotic approach, which are fixed and to be strictly followed by all members, from patient preparing, positioning, port locations, cannulation methods, pericardial opening, clamping aorta, valve exposure and even techniques for valve repair. The standardized steps avoid complications encountered during minimal invasive surgery. Third, team debriefing for every surgery in the beginning stage. In this early stage, we analyzed the videos and debriefings of team members, which helped the team to build up more secure, safe and efficient surgical steps. Fourth, all the robotic cardiac surgeries are performed by the same experience team. Even with the good intra-operative results, the mean hospital stay was 7 days, which is longer than in the experienced centers throughout the Unites States (5,11). That is likely due in part to the health care system in Taiwan, which is a government paid insurance system—the system pays all the admission fees for 30 days after surgery, allowing for longer patient admissions in the general ward until they feel comfortable and then discharged home. We had 2 patients who needed re-checks for bleeding, one from the port site, and the other due to cardioplegic needle aortic root bleeding, all of which can be fixed by thoracoscopic approaches without extending the wound. In the literature, the most common indications for conversion included bleeding, inadequate exposure, patient anatomy, and unsatisfactory repair (12-14). Using standardized techniques, the port location is fixed, and exposure of the mitral valve will not be a problem.
Long-term outcomes and repair durability
We used different mitral valve repair techniques for different mitral leaflet pathologies (Figures 2,3). These techniques were used in all mitral valve repair patients, not only in robotic, but also in conventional repairs for more than 20 years in our center. These can ensure the valve repair feasibility and good repair outcomes. Long-term freedom from re-operation at 10 years was 98% after robotic mitral repair. Ten-year freedom from more than moderate mitral regurgitation in all repair patients was 97.6%. All the repaired valves were secure with full ring annuloplasty using interrupted polyester sutures, which has been established and proved to have long-term durability. Every technique we applied for robotic mitral repair is the same as with a conventional approach, we never compromised on the procedure.
Despite favorable outcomes associated with robotic MV surgery, concerns about procedural safety and cost have limited its acceptance. Patients can obviously benefit from totally endoscopic robotic-assisted approaches given maintenance of thoracic cage integrity, and should have better functional recovery. The procedural safety can be improved upon by training, practice and standardized protocols (18,19). An experienced team setup is important. The team approach should involve all the members in the operation room, CPB technicians, anesthesiologist, nurses, and cardiac surgeons. Everyone should work at the same pace and know every step (6). Training is a basic essential, as robotic instrument control is new for most surgeons. To be familiar how to handle the robotic instruments and understand the limits of the machine requires time and continuous practice. Those efforts can shorten the learning curve and achieve procedural safety.
Conclusions
In conclusion, in an experienced center, excellent short-term outcomes can be obtained in the treatment of severe mitral regurgitation with a totally endoscopic, robotic-assisted approach. The long-term durability of robotic mitral repair can also be achieved by using established valve repair techniques.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chitwood WR Jr. Robotic mitral valve surgery: overview, methodology, results, and perspective. Ann Cardiothorac Surg 2016;5:544-55. [Crossref] [PubMed]
- Chitwood WR Jr. Robotic Mitral Valve Repair: How I Teach It. Ann Thorac Surg 2019;107:1297-301. [Crossref] [PubMed]
- Javadikasgari H, Suri RM, Gillinov AM. Robotic mitral valve repair: algorithmic approach in degenerative mitral valve disease. Ann Cardiothorac Surg 2016;5:586-8. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]
- Suri RM, Dearani JA, Mihaljevic T, et al. Mitral valve repair using robotic technology: Safe, effective, and durable. J Thorac Cardiovasc Surg 2016;151:1450-4. [Crossref] [PubMed]
- Ishii H, Ting M, Chi NH. Robotic mitral valve repair: standardized repair strategy ensures consistent results. Ann Cardiothorac Surg 2018;7:837-8. [Crossref] [PubMed]
- Chou NK, Okano R, Tedoriya T, et al. Robotic Transmitral Approach for Hypertrophic Cardiomyopathy With Systolic Anterior Motion. Circ J 2018;82:2761-6. [Crossref] [PubMed]
- Chi NH, Huang CH, Huang SC, et al. Robotic mitral valve repair in infective endocarditis. J Thorac Dis 2014;6:56-60. [PubMed]
- Wang YC, Chi NH, Wang YC, et al. Retrograde arterial perfusion and its outcome in robotic mitral-valve surgery. Asian J Surg 2022;45:1849-54. [Crossref] [PubMed]
- Chi NH, Fu HY, Yu HY, et al. Comparison of robotic and conventional sternotomy in redo mitral valve surgery. J Formos Med Assoc 2022;121:395-401. [Crossref] [PubMed]
- Loulmet DF, Ranganath NK, Neragi-Miandoab S, et al. Advanced experience allows robotic mitral valve repair in the presence of extensive mitral annular calcification. J Thorac Cardiovasc Surg 2019;S0022-5223(19)32405-5. Epub ahead of print. [Crossref] [PubMed]
- Goodman A, Koprivanac M, Kelava M, et al. Robotic Mitral Valve Repair: The Learning Curve. Innovations (Phila) 2017;12:390-7. [Crossref] [PubMed]
- Gillinov AM, Mihaljevic T, Javadikasgari H, et al. Early results of robotically assisted mitral valve surgery: Analysis of the first 1000 cases. J Thorac Cardiovasc Surg 2018;155:82-91.e2. [Crossref] [PubMed]
- Toolan C, Palmer K, Al-Rawi O, et al. Robotic mitral valve surgery: a review and tips for safely negotiating the learning curve. J Thorac Dis 2021;13:1971-81. [Crossref] [PubMed]
- Loulmet DF, Koeckert MS, Neuburger PJ, et al. Robotic mitral repair for Barlow's disease with bileaflet prolapse and annular calcification using pericardial patch technique. Ann Cardiothorac Surg 2017;6:67-9. [Crossref] [PubMed]
- Chemtob RA, Wierup P, Mick SL, et al. A conservative screening algorithm to determine candidacy for robotic mitral valve surgery. J Thorac Cardiovasc Surg 2022;164:1080-7. [Crossref] [PubMed]
- Naito N, Grossi EA, Nafday HB, et al. Robotic mitral valve repair with complete excision of mitral annular calcification. Ann Cardiothorac Surg 2022;11:545-7. [Crossref] [PubMed]
- Bates MJ, Chitwood WR Jr. Minimally invasive and robotic approaches to mitral valve surgery: Transthoracic aortic crossclamping is optimal. JTCVS Tech 2021;10:84-8. [Crossref] [PubMed]
- Bonatti J, Crailsheim I, Grabenwöger M, et al. Minimally Invasive and Robotic Mitral Valve Surgery: Methods and Outcomes in a 20-Year Review. Innovations (Phila) 2021;16:317-26. [Crossref] [PubMed]







