Robotically assisted mitral valve repair—the string, ruler, and bulldog technique
Introduction
Robotic mitral repair surgery is a well-accepted minimally invasive approach with several established techniques that can be used to achieve adequate repair. One of these techniques is neochordae implantation using expanded polytetrafluoroethylene (ePTFE), first described by O. H. Vetter in 1986 (1). This is a well-known and reliable technique to repair diseased mitral valve leaflets (2-5). In this technique, it is crucial to achieve an accurate measurement of the length of the neochordae to be implanted, and to carry out a meticulous ligation of the artificial chordae to avoid a loose knot (5). However, ePTFE sutures are slippery, as a result the knots may slide when tying, especially when using the da Vinci surgical system (Intuitive Surgical Inc., Sunnyvale, 17 CA, USA).
There are products and techniques to more easily facilitate the implantation of neochordae, such as the Chordarizer (Sumitomo Bakelite Co. Ltd., Tokyo, Japan); Chord-x (Cryolife, Inc., Georgia, USA) and Leipzig Loop technique (6,7). This article presents, in detail, the surgical steps for robotic mitral valve repair utilizing a string, a ruler, and a bulldog vascular clamp to assist with the implantation of neochordae, which is a simple, effective, and reproducible technique using the robotic platform (Video 1).
Patient selection
All patients undergo coronary artery angiogram or computed tomography coronary artery angiogram, transthoracic and transesophageal echocardiogram (TTE, TEE) to accurately define the mitral valve pathology and anatomy, and computed tomography of the aorta to assess feasibility for peripheral access. Contraindications for robotic-assisted mitral valve repair include concomitant cardiac procedure (except tricuspid valve repair/replacement, atrial septum defect), abnormal thoracic anatomy, poor shoulder and/or elbow mobility (8) (Tables 1,2).
Table 1
| Previous right thoracotomy |
| Severe pulmonary dysfunction |
| Myocardial infarction or ischaemia <30 days |
| Coronary artery disease—requiring CABG |
| Severe generalized vascular disease |
| Symptomatic CVD or stroke <30 days |
| Poor right ventricular function |
| Pulmonary hypertension (fixed > torr) |
| Significant aortic stenosis or insufficiency |
| Severe annular calcification (repairs) |
| Severe liver dysfunction |
| Significant bleeding disorders |
CABG, coronary artery bypass grafting; CVD, cerebrovascular disease.
Table 2
| Previous sternotomy |
| Moderate pulmonary dysfunction |
| Asymptomatic CAD (treated) |
| Coronary artery disease—requiring PCI |
| Limited peripheral vascular disease |
| Asymptomatic CVD |
| Poor left ventricular function (EF <30%) |
| Mild to moderate aortic stenosis or insufficiency |
| Moderate annular calcification |
CAD, coronary artery disease; PCI, percutaneous coronary artery intervention; CVD, cerebrovascular disease; EF, ejection function.
Preparation
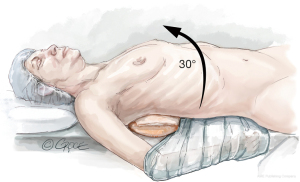
After double lumen intubation, a radial arterial pressure monitoring line, central venous line, internal jugular vein cannula, and pulmonary artery catheter are placed. The patient is positioned in a lazy lateral decubitus, with a soft roll over the right paraspinal muscles, displacing the right hemi-thorax upwards, providing a direct view to the mitral valve. The suprasternal notch, sternomanubrial junction and intercostal spaces (ICS) are identified with a marking pen. An alcohol-based iodine solution is painted on the skin and evaporated to dryness, before the patient is draped widely to allow access to the groin and the right axillary lines. Prior to incision, appropriate prophylactic antibiotics are administered (Figure 1).
Peripheral cannulation
A 3 cm oblique incision is performed in the right groin. A plane is developed through the subcutaneous tissue, with vessels clipped using tritium ligating clips (Weck® Horizon™ Metal Ligation System, USA) and divided. The anterior aspects of the femoral vessels are exposed. 5-0 polypropylene purse string sutures are placed in the common femoral artery and vein. After systematic heparinization, the femoral vessels are cannulated using a Seldinger technique. After puncture of the common femoral artery, a soft guidewire is placed in the descending thoracic aorta under the guidance of TEE. The puncture site is progressively dilated and an arterial femoral cannula of adequate size is inserted and secured. After puncture of the common femoral vein, a soft guidewire is passed up to the superior vena cava (SVC). The wire position is confirmed by TEE using a bicaval view. The puncture site is progressively dilated, and a 25 Fr multi-stage venous cannula (Maquet Getinge Group, Rastatt, Germany) is introduced. The tapered tip insert is not advanced further once it enters the right atrium. Only the venous cannula itself is now advanced forward over the insert, strictly under TEE guidance. It is essential that the cannula tip is placed in the SVC to ensure satisfactory bicaval venous return. The insertion of the cannulas should be without any resistance.
Incision and port placement
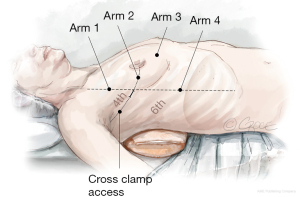
A 3 cm incision is made in the right anterior axillary line in the 4th ICS. The inferior edge of the pectoralis major muscle is dissected using diathermy, allowing for the muscle to retract. Single lung ventilation is commenced, and an “Alexis” wound retractor (Applied Medical, Rancho Santa Margarita, CA, USA) is placed. A port is formed in the 4th ICS with an 8 mm trocar, anterior to the 3 cm working port, to accommodate the 30° angle robotic camera (Arm 2). The thorax is insufflated with CO2 via the sideline connection of the same trocar. An additional three ports are formed using trocars in the 3rd (Arm 1, left hand), 4th (Arm 3, atrial lift retractor) and 6th (Arm 4, right hand) ICS, and the robotic arms are docked (Figure 2). Occasionally, the port for arm 4 is formed in the 5th ICS, if the right hemidiaphragm is elevated. The robotic forceps are inserted into the third ICS port and the left atrial lift retractor is inserted in the 4th submammary port. Interatrial sutures are passed through this port, and when tension is applied, it neatly exposes the left atrium.
Exposure and access
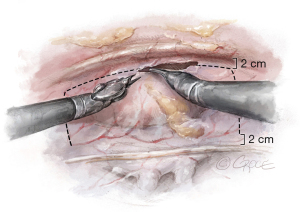
Thymectomy is performed to optimize the surgical field. The pericardium is opened anteriorly, 2 centimeters (cm) from the back of the sternum and is mobilized superiorly at the level of the SVC and inferiorly at the level of the inferior vena cava (IVC), 2 cm away from the phrenic nerve (Figure 3). The pericardial flap is reflected over the lung and secured with two stay sutures, which are brought outside of the thorax with the Endo-Close needle. The angle between the IVC and the left atrium is dissected using blunt and sharp dissection.
A purse-string stitch using a 4-0 polypropylene suture is placed in the ascending aorta to secure a 7-Fr cardioplegic needle (Maquet Cardiopulmonary AG, Germany). The cross-clamp passing through the chest wall is placed in the plane formed between the RPA and aorta, with the custodial cardioplegic solution subsequently administered.
Mitral valve exposure and inspection
Tension is applied to the retracting suture placed in interatrial groove. Left atriotomy is carried out in Sondergaard’s groove, from inferior to superior, followed by the insertion of the left atrial retractor blade. The atriotomy is usually not extended toward the oblique sinus, since the extended atriotomy itself provides adequate exposure. The retractor blade is placed approximately 1 cm from the mitral annulus, and sufficient tension is exerted to expose its posterior aspect (Figure 4). A thorough inspection of the mitral leaflets is carried out.
Neochordae implantation
This comprises of three steps:
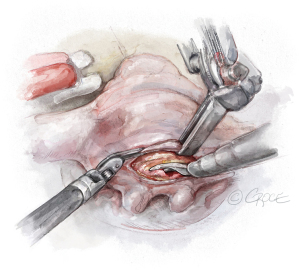
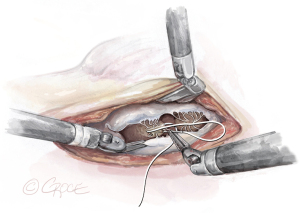
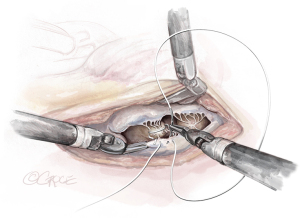
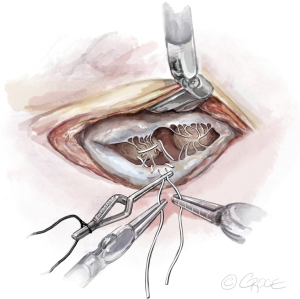
- The String: A substantial papillary muscle is identified. Using an CV4 Gore-Tex suture (GORE-TEX® Suture, W. L. Gore & Associates Inc., Flagstaff, AZ, USA), a figure-of-eight stitch is passed through the fibrous component of the papillary muscle. The same needle is then passed twice through the prolapsing edge of the leaflet. The same steps are repeated with the second needle of the suture. Good purchase of tissues is needed, so the leaflet edge does not tear (Figures 5-7
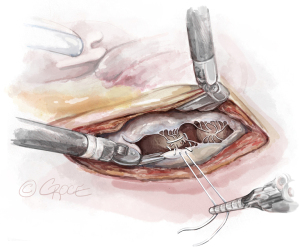
). - The Ruler: A ruler is pre-fashioned to the exact chordae length in accordance with the pre-operative TEE measurements. The ruler is positioned between the papillary muscle and the free edge of the mitral leaflet, so the length of the neochord can be adjusted accordingly (Figure 8).
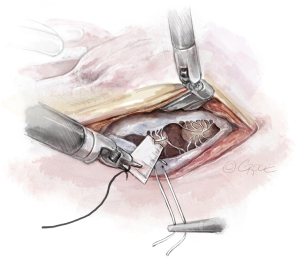
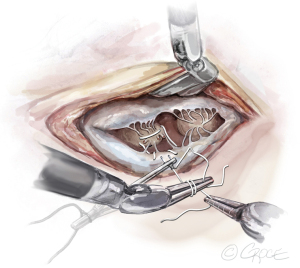
- The Bulldog: A bulldog vascular clamp is applied to both arms of the ePTFE suture. The clamp prevents the knots sliding whilst tying the suture. The bulldog clamp is easily manipulated by robotic needle holders, allowing millimetric adjustments to the length of the neochordae. During tying, it is important to avoid over stretching the corners of the atriotomy. Eight to ten knots are thrown, including square knots to prevent slipping (Figures 9,10
).
Ring size selection, implantation, and valvular testing
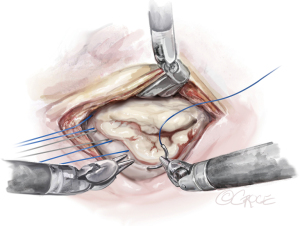
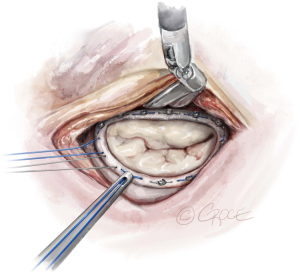
The anterior mitral leaflet is unfurled, and its dimensions are measured to estimate the size of the band to be implanted. In most cases, a Cosgrove Band (Edwards Lifesciences, CA, USA) is used. Interrupted stitches using 2-0 braided polyester sutures are passed through the mitral annulus and then to the annuloplasty band (Figure 11). Here is a good example of how the robotic platform allows surgeons to work ambidextrously—the right arm is used to pass the sutures through the posterior annulus, while left arm is used for the sutures of the anterior annulus. The band is then fastened down using Cor-Knots (Minogue Medical Inc., Quebec, Canada) (Figure 12). After completion of the mitral valve annuloplasty, normal saline is injected into the left ventricle to test the valve.
Completion
The left atriotomy is closed in two layers using a 3-0 polypropylene suture. A bipolar temporary pacing wire is inserted in the epicardium of the right ventricle, which is brought out to the skin via the cross-clamp port. Normal hemodynamic function is restored, and TEE analysis of the mitral valve repair is performed. Hemostasis is carefully conducted, and the patient is weaned from cardiopulmonary bypass (CPB) and decannulated. Two 32-Fr soft drains (BLAKE® Silicone Drains, Ethicon, USA) are placed in the right thorax. A No. 1 Vicryl suture is used to close the fascia and the subcutaneous fat. The skin is closed with a 5-0 Monocryl subcuticular suture. This completes the robotic assisted mitral valve repair using a string, a ruler, and a bulldog clamp.
Discussion
The technique described herein is simple and reproducible. There are other techniques of neochordae implantation designed for minimal access surgery, such as the Leipzig Loop technique, Chordarizer (Sumitomo Bakelite Co. Ltd., Tokyo, Japan) and Chord-x (Cryolife, Inc., Georgia, USA).
In the Leipzig Loop technique, the required length of the neochordae is measured against an adjacent non-prolapsing segment and the respective papillary muscle by using a ruler or measuring device (03-5409; Geister, Tuttlingen, Germany), or by transesophageal echocardiography. The CV5 ePTFE (Gore-Tex) loops are formed by going around the arms of the vernier caliper, and tied over a pledget to retain the “premeasured” length. The needles are then passed anterior to posterior on the respective papillary muscle and tied over a second pledget. The tip of the loop is then secured to the edge of the leaflet using a second suture (7). While using the Chordarizer device, the ePTFE is passed through the papillary muscle in an over-and-over fashion. The nearby normal chordae are measured with a specific Chordarizer ruler. The correct size Chordarizer is selected and locked in place around both arms of the ePTFE suture. The sutures are passed through the free edge of the diseased segment of the mitral leaflet and secured over the Chordarizer, which acts as stop block (6). The implantation of the Chord-X, a pre-manufactured artificial chordae, is similar to the Leipzig Loop technique. The main difference is that the neochordae are secured at the free mitral valve leaflet edge using the same suture.
The main differences/advantages of the technique described when compared to the above are: (I) it does not require special tools for neochordae implantation; (II) does not require knot tying in the area of the subvalvular apparatus, and is therefore less likely to cause damage to it and atrial wall; (III) precise length of neochordae can be achieved with the ruler; (IV) reliable knot tying and retention of the exact neochordae length with the bulldog clamp. In general, if the structural causes of mitral regurgitation are fully understood, an accurate pre-operative measurement of the normal chordae is obtained, the playbook is followed and the mechanical problems of the mitral valve are fixed accordingly, one will achieve a robust mitral valve repair. Furthermore, a surgeon may have a better chance to achieve meticulous repair using the robotic approach, due to its unparalleled 3-dimensional visualization and enhanced bi-manual motor coordination.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vetter OH, Burack JH, Factor SM, et al. Replacement of chordae tendineae of the mitral valve using the new expanded PTFE suture in sheep. In: Bodnar E, Yacoub M. editors. Biologic and Bioprosthetic Valves. New York: Yorke Medical Books; 1986:772-85.
- Seeburger J, Kuntze T, Mohr FW. Gore-tex chordoplasty in degenerative mitral valve repair. Semin Thorac Cardiovasc Surg 2007;19:111-5. [Crossref] [PubMed]
- Bizzarri F, Tudisco A, Ricci M, et al. Different ways to repair the mitral valve with artificial chordae: a systematic review. J Cardiothorac Surg 2010;5:22. [Crossref] [PubMed]
- Bortolotti U, Milano AD, Frater RW. Mitral valve repair with artificial chordae: a review of its history, technical details, long-term results, and pathology. Ann Thorac Surg 2012;93:684-91. [Crossref] [PubMed]
- Hata H, Fujita T, Shimahara Y, et al. A 25-year study of chordal replacement with expanded polytetrafluoroethylene in mitral valve repair†. Interact Cardiovasc Thorac Surg 2015;20:463-8; discussion 468. [Crossref] [PubMed]
- Tedoriya T, Okano R, Fukuzumi M, et al. A simple technique of artificial chordae implantation in robotic cardiac surgery using a novel tube device supporting expanded polytetrafluoroethylene chordae ligation. Eur J Cardiothorac Surg 2021;60:189-90. [Crossref] [PubMed]
- von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg 2000;70:2166-8. [Crossref] [PubMed]
- Chitwood WR Jr. Robotic mitral valve surgery: overview, methodology, results, and perspective. Ann Cardiothorac Surg 2016;5:544-55. [Crossref] [PubMed]






