Hybrid uniportal robotic-assisted thoracoscopic surgery using video-assisted thoracoscopic surgery staplers: technical aspects and results
Introduction
The advent of surgical robots has completely revolutionized the treatment of early-stage lung cancer. Since the very first report of surgical robots used for computed tomography (CT)-guided brain tumor biopsies in 1985 (1), robotic technologies have been widely used in surgeries owing to their stability, maneuverability and accuracy. Recent technical advances have made it increasingly possible for robots to overcome the operative limitations of traditional thoracoscopic surgery. Melfi et al. reported the initial experience of thoracic surgeries conducted through a robotic system (2). Numerous studies thereafter comprehensively evaluated the clinical efficacy of robot-assisted thoracic surgeries (3-6). Nonetheless, current standard robotic systems were intended for multiportal surgical access, hence obstacles still exist concerning the feasibility of single-port robotic surgeries.
In 2019, Gonzalez-Rivas initially conducted a cadaveric experiment to evaluate the feasibility of the uniportal robotic system, the da Vinci SP system (Intuitive Surgical, Inc., Sunnyvale, CA 94086, USA) (7). Subsequently, a uniportal robot-assisted thoracoscopic surgery (U-RATS) approach (da Vinci Xi system) utilizing robotic staplers was developed and complex resections including sleeve resections and pneumonectomies were successfully carried out (8). Nonetheless, since robotic staplers for U-RATS were recently invented and have not been widely applied among institutions, especially in the developing world, hybrid U-RATS utilizing video-assisted thoracoscopic surgery (VATS) staplers might serve as an effective substitute. Based on this notion, Yang et al. reported a hybrid surgery modality also using the da Vinci Xi system and elucidated its potential in thoracic surgeries (9). However, more evidence is needed to further demonstrate the clinical efficacy of such a surgical modality.
In this single-center retrospective study, we reported the specific technical skills of hybrid U-RATS using VATS staplers conducted recently in Shanghai Pulmonary Hospital and the early perioperative outcomes of 40 cases. The clinical efficacy of hybrid U-RATS was preliminarily validated.
Methods
Study design
This study was a single-institution retrospective study reporting the technical aspects and early outcomes of hybrid U-RATS using VATS staplers, which was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived by the Institutional Review Board of Shanghai Pulmonary Hospital due to its retrospective nature. Electronic records of the patients with indeterminant pulmonary nodules, and afterwards receiving hybrid U-RATS between August 2022 and September 2022 were collected. These two months were randomly selected to better illustrate the clinical efficacy of the hybrid procedure. Patients who had received neoadjuvant therapies or with missing data were excluded. All the surgical procedures were conducted by Dr. Zhao. Clinicopathological characteristics of these patients were afterwards collected including baseline information [comorbidities were assessed using Charlson Comorbidity Index (CCI) (10)] and pathological results. Histological subtypes were classified in accordance with the 2021 World Health Organization (WHO) classification system (11), and the pathological stages were evaluated using the eighth edition of the tumor node metastasis (TNM) staging system (12).
Surgical procedures
Hybrid U-RATS was conducted using the da Vinci Surgical Xi system (Intuitive Surgical, Inc., 1266 Kifer Road, Sunnyvale, CA 94086, USA), which has been certified and applied at our institution since October 2020. A single, 4- to 5-cm-long incision was customarily made at the posterior axillary line at the 5th intercostal space and then expanded. After the central point of the da Vinci system was aligned with the incision, robotic arms were then rotated to coincide with the longitudinal axis of the patient’s body. Three robotic arms (arms 1, 2 and 3 or arms 2, 3 and 4) were generally selected during the operation (Figure 1). The camera arm was usually placed to the upper edge of the incision using arm two, and monopolar or bipolar forceps were afterwards placed close to arm two using arm three. The electrocautery hook was then placed close to the lower edge of the incision by arm four. Spaces between arms three and four would also allow the assistant to employ oval forceps or a suction device to assist the exposure process.
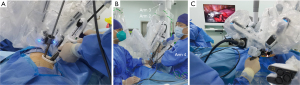
During the hybrid surgical process, lymph nodes were removed through the all-round rotation of the electrocautery hook under the assistance of the robotic arm after local tension formation. With the removal of the lymph nodes, the hilar structure could be fully exposed. After lymph node dissection, robotic arm four would then be retracted out, and the VATS stapler would be extended straight into the thoracic cavity, and adjusted according to the position of the hilar structures. The exposed structures would then be divided consecutively. The resection range would be determined based on the results of the intraoperative frozen sections.
Perioperative outcomes evaluation
Intraoperatively collected parameters comprised of operation duration, blood loss volume and event of conversion to open access. Other post-operative parameters including hospital length of stay (LOS), drainage duration and volume, extra analgesic administration status, readmission and mortality status were also collected. Postoperative complications were evaluated and graded using the Clavien-Dindo classification criteria (13).
Statistical analysis
Categorical variables were expressed as numbers with percentages, while continuous variables were expressed as the median with interquartile range (IQR). All the statistical analyses were performed through SPSS (version 26, IBM Corp., Armonk, NY, USA), and all the graphs were created using GraphPad Prism (version 9.0, Dotmatics, CA, USA). All the P values were two-sided with a statistical significance level of <0.05.
Results
Clinicopathological characteristics
A total of 40 patients were included in this study (Table 1), with a median age of 63 years (IQR, 52–71 years). Nineteen (47.5%) and 21 (52.5%) patients were male and female, respectively. The median BMI of all the patients was 23.1 kg/m2 (IQR, 21.3–25.8 kg/m2). Only four patients (10.0%) were current smokers or ever smoked. Half of the patients had a CCI score of 1–2. Concerning the pathological results, most of the patients (31/40, 77.5%) were diagnosed with one lesion, with adenocarcinoma being the prominent histological subtype (33/40, 82.5%). All the surgical margins (40 cases) were evaluated free of tumor cells. Based on the TNM staging system, three (7.5%), 30 (75.0%), three (7.5%), and two (5.0%) patients were evaluated as pathological stage 0, I, II, and III, respectively. Eight (20.0%) specimens were also evaluated for presence of “spread through air spaces” (STAS), which was also an important indicator for prognosis. Among the patients with dissected lymph nodes (37 cases), the median number of lymph nodes dissected was 11 (IQR, 7-15), and the median station number dissected was six (IQR, 5–6) (Table S1). A total of three nodal upstaging events were encountered, with each of them separately upstaged from cN0 to pN1, from cN0 to pN2 and from cN1 to pN2.
Table 1
| Characteristics | Total (n=40) |
|---|---|
| Age (years), median [IQR] | 63 [52–71] |
| Sex, n (%) | |
| Male | 19 (47.5) |
| Female | 21 (52.5) |
| BMI (kg/m2), median [IQR] | 23.1 [21.3–25.8] |
| Smoking history, n (%) | |
| Current or ever | 4 (10.0) |
| Never | 36 (90.0) |
| CCI, n (%) | |
| 0 | 6 (15.0) |
| 1–2 | 20 (50.0) |
| ≥3 | 14 (35.0) |
| Number of lesions, n (%) | |
| 1 | 31 (77.5) |
| 2 | 9 (22.5) |
| Histological classification, n (%) | |
| Adenocarcinoma | 33 (82.5) |
| AIS or MIA | 11 (27.5) |
| Invasive adenocarcinomaa | 22 (55.0) |
| Grade I | 2 (5.0) |
| Grade II | 13 (32.5) |
| Grade III | 7 (17.5) |
| Squamous cell carcinoma | 4 (10.0) |
| Large cell carcinoma | 1 (2.5) |
| Benign lesion | 2 (5.0) |
| Pathological T stageb, n (%) | |
| pTis | 3 (7.5) |
| pT1a (mi) | 7 (17.5) |
| pT1a | 5 (12.5) |
| pT1b | 10 (25.0) |
| pT1c | 5 (12.5) |
| pT2a | 6 (15.0) |
| pT2b | 1 (2.5) |
| pT3 | 1 (2.5) |
| NAc | 2 (5.0) |
| Pathological N stageb, n (%) | |
| pN0 | 35 (87.5) |
| pN1 | 1 (2.5) |
| pN2 | 2 (5.0) |
| NAc | 2 (5.0) |
| Pathological TNM stageb, n (%) | |
| 0 | 3 (7.5) |
| IA1 | 12 (30.0) |
| IA2 | 9 (22.5) |
| IA3 | 5 (12.5) |
| IB | 4 (10.0) |
| IIA | 1 (2.5) |
| IIB | 2 (5.0) |
| IIIA | 2 (5.0) |
| NAc | 2 (5.0) |
| STAS presence, n (%) | 8 (20.0) |
a, all the invasive adenocarcinomas were graded according to the IASLC grading system; b, pathological stage was classified according to the eighth edition of the TNM classification; c, two benign lesions were not applicable for the pathological stage evaluation. RATS, robot-assisted thoracoscopic surgery; IQR, interquartile range; BMI, body mass index; CCI, Charlson comorbidity index; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; TNM, tumor node metastasis; STAS, tumor spread through air spaces; NA, not available.
Perioperative outcomes
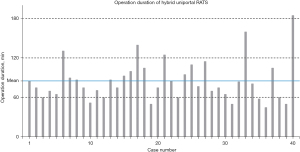
All the hybrid surgical processes were conducted through a single, intercostal access. Lobectomies were the most frequently conducted operations (23/40, 57.5%) in this cohort, while bilobectomies, segmentectomies and wedge resections we also carried out less frequently (Table 2). Notably, there was one patient having received a bronchial sleeve lobectomy. One conversion from U-RATS to a biportal approach was encountered due to extensive adhesions discovered intraoperatively, which were hard to tackle through a uniportal approach. The median procedural duration of the entire cohort was 76 min (IQR, 61–99 min) (Figure 2), and the median intraoperative blood loss volume was 50 mL (IQR, 50–50 mL). No vessel injury or hemorrhage was observed by the stapler passing through vessels. The median LOS was three days (IQR, 2–4 days), and the median drainage duration was also three days (IQR, 2–4 days). A median drainage volume of 263 mL (IQR, 155–491 mL) was recorded. Eleven patients (27.5%) experienced postoperative complications including pneumonia, transient elevation of serum creatinine and atrial fibrillation graded as Clavien-Dindo I–II, while none of the patients experienced grade III–IV complications. Six patients (15.0%) were administered with extra analgesia. None of the patients were readmitted or died from causes related to the operation within 30 days post-surgery.
Table 2
| Characteristics | Total (n=40) |
|---|---|
| Resection range, n (%) | |
| Bilobectomy | 1 (2.5) |
| Lobectomy + wedge resection | 2 (5.0) |
| Segmentectomy + wedge resection | 3 (7.5) |
| Lobectomy | 23 (57.5) |
| Bronchial sleeve lobectomy | 1 (2.5) |
| Segmentectomy | 10 (25.0) |
| Conversion to biportal access, n (%) | 1 (2.5) |
| Operation duration (min), median [IQR] | 76 [61–99] |
| Intraoperative blood loss (mL), median [IQR] | 50 [50–50] |
| LOS (days), median [IQR] | 3 [2–4] |
| Drainage duration (days), median [IQR] | 3 [2–4] |
| Drainage volume (mL), median [IQR] | 263 [155–491] |
| Postoperative complicationsa, n (%) | |
| Clavien-Dindo I–II | 11 (27.5)b |
| Pneumonia | 6 (15.0) |
| Transient elevation of serum creatinine | 5 (12.5) |
| Atrial fibrillation | 2 (5.0) |
| Clavien-Dindo III–IV | 0 (0.0) |
| Administration of extra analgesic, n (%) | 6 (15.0) |
| Readmission within 30 days, n (%) | 0 (0.0) |
| Mortality within 30 days, n (%) | 0 (0.0) |
a, postoperative complications were evaluated in accordance with Clavien-Dindo classification; b, one patient experienced both transient elevation of serum creatinine and pneumonia, and another patient also experienced both transient elevation of serum creatinine and atrial fibrillation. RATS, robot-assisted thoracoscopic surgery; IQR, interquartile range; LOS, length of stay.
Discussion
Surgical resection serves as an effective treatment modality for early-stage non-small cell lung cancer (14). Recent advances in mechanics, automation, and engineering have made it possible for robots to be applied in thoracic surgeries. Through remote instrumental control, data transmission and three-dimensional (3D) imaging, robotic surgeries have been validated to achieve comparable perioperative outcomes and long-term efficacy to the well-established video-assisted thoracoscopic surgeries (4,5,15,16). Concerning the status quo of robotic surgeries, the multiportal approach through the da Vinci Xi system is currently among the most frequently reported modalities. Based on the notion of developing a uniportal approach, Gonzalez-Rivas et al. recently reported the early experience of conducting U-RATS using robotic staplers (8). Nonetheless, since a dedicated uniportal robotic system and robotic staplers are still not available in the developing world, Yang et al. reported a combination modality of uniportal robotic system with VATS stapler and initially verified its feasibility (9). Based on the notion of taking the advantages from both RATS and uniportal access, we also carried out hybrid U-RATS using VATS staplers at our institution. The results from the recent 40 cases have revealed that such a procedure harbored comparable clinical efficacy to traditionally adopted video-assisted thoracic surgeries and profound potential for future application.
Yang et al. specifically described the operative techniques of hybrid U-RATS in their research (9). Based on our preliminarily experience in conducting hybrid surgeries using VATS staplers, several comments concerning this technique could be drawn. Firstly, the incision was generally made at the posterior axillary line at the 5th intercostal space, not too close to the midline or at the lower intercostal space, which can help to avoid interference between the robotic arms. Secondly, when operating the robotic arms, it would further reduce the interference between trocars if arm 3, which was commonly close to arm 2 near the upper edge of the incision, and arm 4, which was commonly close to the lower edge of the incision, were spread out and extended into the thoracic cavity at different depths. Thirdly, it would not be difficult to recognize the advantages in clinical practice of hybrid U-RATS, that is the thorough removal of the lymph nodes and anatomical dissection at the hilum would allow the placement of VATS stapler without any difficulty.
Owing to the fact that operations dissecting the peri-hilar structures through video-assisted thoracoscopic surgeries were customarily one-directional, procedures of the hybrid U-RATS reported here were difficult to achieve through VATS. Another advantage brought by the robotic system was that the anatomical structure could be clearly defined before the dissection without misjudging important structures, and the time consumption for extending or extracting the stapler was further reduced. Concerning the learning curve of this procedure, despite corresponding data were still lacking, based on the experience acquired at our institution, we would speculate that for surgeons mastering multiportal RATS, 20–30 cases were needed to get accustomed to single-port RATS, and probably another 20–30 cases were still needed to master hybrid U-RATS.
Several limitations are apparent concerning this study. Firstly, due to its single-center retrospective nature, short study duration, and the limited sample size, selection bias may interfere with the results. Since this study was a single-arm study, no comparison between the hybrid U-RATS and the established uniportal VATS or multiportal RATS was conducted. Future studies are still warranted to further demonstrate the clinical efficacy of such a procedure. Lastly, survival outcomes of the hybrid U-RATS are still needed to illustrate the long-term efficacy. As there was no precise volumetric measuring equipment in the operating room, volume loss was estimated to be 50mL; as such, the IQR is presented as 50–50 mL. This will be a point of focus in additional studies to more accurately reflect perioperative blood loss.
Conclusions
In summary, the procedure of hybrid U-RATS using the VATS stapler has been evaluated to be feasible and safe. Based on the analyses of the early outcomes of this modality, the clinical efficacy of the hybrid U-RATS has been preliminarily verified. Such a hybrid procedure harbors profound potential in the surgical treatment strategies of early-stage non-small cell lung cancer.
Acknowledgments
Funding: This study was supported by National Key R&D Program of China (Nos. 2021YFC2500904 and 2021YFC2500905), Shanghai Hospital Development Center (Nos. SHDC22021310-A and SHDC22021217), and Shanghai Municipal Health Commission (No. 202040322).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kwoh YS, Hou J, Jonckheere EA, et al. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng 1988;35:153-60. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Zhao X, Qian L, Lin H, et al. Robot-assisted lobectomy for non-small cell lung cancer in china: initial experience and techniques. J Thorac Dis 2010;2:26-8.
- Qian L, Chen X, Huang J, et al. A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis 2017;9:1997-2005. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Jiao W, Zhao Y, Qiu T, et al. Robotic Bronchial Sleeve Lobectomy for Central Lung Tumors: Technique and Outcome. Ann Thorac Surg 2019;108:211-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Ismail M. Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience. J Thorac Dis 2019;11:231-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Motas N, et al. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg 2022;62:ezac410. [Crossref] [PubMed]
- Yang Y, Song L, Huang J, et al. A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) Surgical System in the treatment of early-stage lung cancer. Transl Lung Cancer Res 2021;10:1571-5. [Crossref] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Lang-Lazdunski L. Surgery for nonsmall cell lung cancer. Eur Respir Rev 2013;22:382-404. [Crossref] [PubMed]
- Li C, Hu Y, Huang J, et al. Comparison of robotic-assisted lobectomy with video-assisted thoracic surgery for stage IIB-IIIA non-small cell lung cancer. Transl Lung Cancer Res 2019;8:820-8. [Crossref] [PubMed]
- Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann Surg 2022;275:295-302. [Crossref] [PubMed]






