Uniportal robotic versus thoracoscopic assisted surgery: a propensity score-matched analysis of the initial 100 cases
Introduction
Minimally invasive surgery (MIS) is currently considered the gold-standard technique for major pulmonary resections instead of conventional thoracotomy due to better perioperative outcomes with equivalent oncological efficacy (1-5).
In particular, since Gonzalez reported the first uniportal video-assisted thoracic surgery (uVATS) lobectomy in 2011 (6), this approach has progressively gained relevance in thoracic surgery units. Several studies have suggested that uVATS offers potential benefits compared to multiportal video-assisted thoracic surgery (mVATS), including less postoperative pain and a direct view from a sagittal perspective (7-13). The fact that surgeons work with their eyes and hands in the same plane has helped to perform complex procedures in locally advanced lung cancer, like pneumonectomy, chest wall resections, bronchovascular sleeves and carinal reconstructions (14-16). In contrast, crowding all the instrumentation through the same incision can compromise instrument flexibility and limit range of movement, requiring time to master (17,18).
Meanwhile, multiportal robotic-assisted thoracic surgery (mRATS) experience has demonstrated technical advantages such as three-dimensional view, camera stability and improved maneuverability due to instruments with seven degrees of freedom and articulations that mimic human finger joints without physiological tremors (19-23). However, it usually requires the employment of four or five ports, which contradicts the basic concept of minimal invasiveness.
Blending the benefits of the uniportal approach and robotic technology, Gonzalez-Rivas adapted the da Vinci Surgical System Xi® to perform the first uniportal robotic-assisted thoracic surgery (uRATS) lobectomy in September 2021. He defined pure uRATS as robotic thoracic surgery performed by a single intercostal incision without rib spreading, using the robotic camera, robotic dissecting instruments and robotic staplers (24,25).
This study performs a propensity score-matched (PSM) analysis comparing the short-term outcomes between uVATS and uRATS in the initial 100 cases of anatomical pulmonary resections.
Methods
Study design & data collection
A retrospective review of a prospectively maintained database identified 200 patients who underwent anatomic lung resections by the same surgeon from August 2010 to October 2022, including the initial 100 cases of uVATS and the initial 100 cases of uRATS. The decision of the approach for each patient was at the discretion of the surgeon’s experience. All patients signed a standard informed consent of data-use agreement approved by their admitting hospital.
The preoperative workup involved routine blood examinations, pulmonary function tests, cardiological assessment, thoracic-abdomen-pelvic computed tomography and total body positron emission tomography.
The variables studied in each patient included:
- Clinical variables:
- Demographic features: gender, age, smoking habit, forced expiratory volume in the first second (FEV1) and cardiovascular risk factors (CVRF).
- Characteristics of the surgical procedure: pleural adhesions, type of resection (segmentectomy, lobectomy, bilobectomy, pneumonectomy and airway resection) and associated sleeve technique. Segmentectomies were categorized as simple or complex. Simple segmentectomies were considered left upper tri-segmentectomy, lingulectomy, S6 segmentectomy or basilar segmentectomy. Complex segmentectomies were considered as any single and/or multiple individual segmentectomies not included as simple.
- Anatomopathological findings: tumor size, histology and TNM classification in cases of non-small cell lung cancer (NSCLC).
- Variables results:
- Operative outcomes: surgical time, intraoperative complication, type of intraoperative complication (arrhythmia, bleeding, endogia failure, inadequate anatomic structure section), conversion to thoracotomy, number of resected lymph nodes and number of nodal stations explored.
- Thirty-day postoperative outcomes: postoperative complication, grading of complication following the Ottawa Thoracic Morbidity and Mortality (TM&M) classification system described in Table 1 (26), opioid usage, prolonged air leak defined as an air leak for over five days, duration of chest drain, length of intensive care unit (ICU) stay, length of hospitalization, reintervention, cause of reintervention (bleeding, air leak, chylothorax, fistula) and mortality rate.
Table 1
| Grading | Definition |
|---|---|
| Minor | |
| Grade 1 | Pharmacologic treatment or other intervention are not required |
| Grade 2 | Pharmacologic treatment or minor intervention required |
| Major | |
| Grade 3a | Surgical, radiologic, endoscopic treatment or multitherapy required without general anesthesia |
| Grade 3b | Surgical, radiologic, endoscopic treatment or multitherapy required with general anesthesia |
| Grade 4a | Intensive care unit treatment for single organ dysfunction required |
| Grade 4b | Intensive care unit treatment for multiple organ dysfunction required |
| Mortality | |
| Grade 5 | Adverse event which leads to death |
Complications* = any deviation from the normal postoperative course. TM&M, grading of postoperative complications following the TM&M classification system.
Surgical technique
Surgical technique is described in Figures 1,2. For both groups, patients received general anesthesia with double-lumen endotracheal intubation and were positioned in the full lateral decubitus position. All surgical instruments were placed through a single port without rib spreading, and a wound protector was routinely used. For uVATS, a 3–4 cm incision was placed in the 4th–5th intercostal space (ICS) between the anterior and mid-axillary lines. Surgery was performed using a 30º camera and uVATS type instruments (grasper, dissector, stapler and suction) (27). For uRATS, a 4 cm incision was placed in the 6th–7th ICS between the anterior and mid-axillary lines. Surgery was performed using three robotic arms (30º robotic camera, robotic hand instruments and robotic staplers) and two uVATS instruments (suction and grasper with subxiphoid length) (25,28).
Statistical analysis
To create a comparable group of patients and minimize bias caused by the non-randomized allocation of treatments, a PSM analysis was performed. The propensity score was calculated by applying a multivariable logistic regression model including gender, age, smoking habit, FEV1, CVRF, pleural adhesions and tumor size. Patients were matched using the nearest neighbor matching method without replacement: a one-to-one ratio with the closest estimated propensity score on the logit scale. A caliper was set to indicate the maximum distance at which two patients could be matched. The caliper was 25% of the standard deviation from the estimated propensity index. Finally, 136 patients, including 68 who underwent uVATS and 68 who underwent uRATS, were enrolled in the study. Continuous data were expressed as mean ± standard deviations, while categorical variables were presented as frequencies and percentages. A comparison between the two groups was carried out before and after PSM analysis. The independent sample t-test was used for continuous variables, whereas χ2 or Fisher exact test was used for categorical variables. Stata software (v. 14.2 for Mac; TX 77845, USA) was employed for all statistical analyses. Statistical significance was defined as P values <0.05.
Results
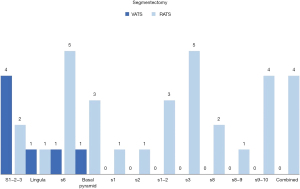
Clinical characteristics of the study are listed in Table 2. After PSM analysis, gender, smoking habit, FEV1, CVRF and pleural adhesions were comparable for both groups. Significant differences between uVATS and uRATS were found concerning the type of resection (P<0.001): the distribution was segmentectomy (6% vs. 29%), lobectomy (80% vs. 59%), bilobectomy (4% vs. 7%), pneumonectomy (10% vs. 2%) and exclusive airway resection (0% vs. 3%). Complex segmentectomy was significantly lower in uVATS than in uRATS (0% vs. 65%, P=0.003). The most common segmentectomy performed by uVATS was left upper tri-segmentectomy and the most common segmentectomy for uRATS was s6 and s3 segmentectomy (Figure 3). The sleeve technique was also significantly lower in uVATS than in uRATS (7% vs. 19%, P=0.043). There were no significant differences regarding the sleeve type. Significant differences between uVATS and uRATS were detected according to the histology (P≤0.001): the distribution was lung cancer (94% vs. 75%), metastasis (4% vs. 12%), benign tumor (2% vs. 7%) and infections (0% vs. 6%). In patients with lung cancer, the TNM stage was similar for both groups.
Table 2
| Characteristics | All patients | Propensity-matched patients | |||||
|---|---|---|---|---|---|---|---|
| uVATS (n=100) | uRATS (n=100) | P | uVATS (n=68) | uRATS (n=68) | P | ||
| Demographic | |||||||
| Gender | |||||||
| Male | 68 (68%) | 60 (60%) | 0.239 | 43 (63%) | 44 (65%) | 0.858 | |
| Female | 32 (32%) | 40 (40%) | 25 (37%) | 24 (35%) | |||
| Age (years) | 66.1±0.9 | 59.4±1.6 | <0.001 | 64.8±1.2 | 65.7±1.1 | 0.573 | |
| Smoking | 73 (73%) | 53 (53%) | 0.003 | 48 (69%) | 47 (69%) | 0.852 | |
| FEV1 (%) | 85.9±2.1 | 86.5±1.3 | 0.808 | 84.4±2.4 | 85.3±1.7 | 0.752 | |
| CVRF | 59 (59%) | 49 (49%) | 0.156 | 40 (59%) | 38 (55%) | 0.729 | |
| Surgical | |||||||
| Adhesions | 40 (40%) | 26 (26%) | 0.035 | 23 (34%) | 22 (32%) | 0.855 | |
| Resection | |||||||
| Segmentectomy* | 7 (7%) | 32 (32%) | <0.001 | 4 (6%) | 20 (29%) | <0.001 | |
| Lobectomy | 80 (80%) | 58 (58%) | 54 (80%) | 40 (59%) | |||
| Bilobectomy | 5 (5%) | 5 (5%) | 3 (4%) | 5 (7%) | |||
| Pneumonectomy | 8 (8%) | 3 (3%) | 7 (10%) | 1 (2%) | |||
| Airway resection | 0 (0%) | 2 (2%) | 0 (0%) | 2 (3%) | |||
| Segmentectomy | |||||||
| Simple | 7 (100%) | 11 (34%) | 0.002 | 4 (100%) | 7 (35%) | 0.003 | |
| Complex | 0 (0%) | 21 (66%) | 0 (0%) | 13 (65%) | |||
| Sleeve | 6 (6%) | 20 (20%) | 0.003 | 5 (7%) | 13 (19%) | 0.043 | |
| Bronchial | 5 | 17 | 0.120 | 5 | 11 | 0.161 | |
| Arterial | 1 | 0 | 0 | 0 | |||
| Double | 0 | 3 | 0 | 2 | |||
| Anatomopathological | |||||||
| Tumor (cm) | 3.1±1.9 | 3.4±2.3 | 0.316 | 3.1±0.2 | 3.0±0.2 | 0.958 | |
| Histology | |||||||
| Lung cancer | 94 (94%) | 70 (70%) | <0.001 | 64 (94%) | 51 (75%) | 0.012 | |
| Metastasis | 5 (5%) | 12 (12%) | 3 (4%) | 8 (12%) | |||
| Benign tumor | 1 (1%) | 8 (8%) | 1 (2%) | 5 (7%) | |||
| Infections | 0 (0%) | 9 (9%) | 0 (0%) | 4 (6%) | |||
| Malformations | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | |||
| TNM for NSCLC | |||||||
| IA1 | 3 (3%) | 0 (0%) | 0.433 | 2 (3%) | 0 (0%) | 0.871 | |
| IA2 | 16 (18%) | 16 (26%) | 14 (22%) | 13 (30%) | |||
| IA3 | 15 (17%) | 5 (8%) | 11 (17%) | 5 (11%) | |||
| IB | 12 (13%) | 8 (13%) | 6 (10%) | 6 (14%) | |||
| IIA | 5 (5%) | 7 (12%) | 3 (5%) | 5 (12%) | |||
| IIB | 18 (20%) | 13 (21%) | 12 (19%) | 8 (19%) | |||
| IIIA | 18 (20%) | 10 (17%) | 12 (19%) | 5 (12%) | |||
| IIIB | 2 (2%) | 2 (3%) | 2 (3%) | 1 (2%) | |||
| IVa | 2 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | |||
Discrete data are expressed as number with percentages: n (%); continuous data are expressed as mean ± SD. Segmentectomy*: anatomic segmentectomy. uVATS, uniportal video-assisted thoracic surgery; uRATS, uniportal robotic-assisted thoracic surgery; FEV1, forced expiratory volume in the first second; CVRF, cardiovascular risk factors; NSCLC, non-small cell lung cancer.
Perioperative outcomes are described in Table 3. After PSM analysis, surgical time, intraoperative complication rate and conversion rate were comparable for both groups. Significant differences were observed concerning the type of operative complication: bleeding was more frequent in the uVATS group and arrhythmia in the uRATS group. There was no surgical mortality in either of the two groups. Regarding lymphadenectomy, the number of resected lymph nodes was significantly lower in uVATS than in uRATS (13.7 vs. 17.6, P=0.007) but the number of nodal stations explored was similar. Postoperative complication rate was higher in uVATS than in uRATS (28% vs. 9%, P=0.004), but the grade of postoperative complications following the TM&M classification system was similar. Neither group had significant differences regarding opioid usage or prolonged air leak. Duration of chest drain was significantly higher in uVATS than in uRATS (3.9 vs. 2.6 days, P=0.034). Length of ICU stay and hospitalization, reintervention rate and mortality rate were similar. Only one patient with relevant comorbidities died after sleeve bilobectomy in the context of acute respiratory distress syndrome.
Table 3
| Variables | All patients | Propensity-matched patients | |||||
|---|---|---|---|---|---|---|---|
| uVATS (n=100) | uRATS (n=100) | P | uVATS (n=67) | uRATS (n=67) | P | ||
| Operative | |||||||
| Time (min) | 144.6±7.4 | 130.3 ±5.6 | 0.127 | 143.9±9.0 | 127.7±7.4 | 0.155 | |
| Complication rate | 6 (6%) | 4 (4%) | 0.748 | 4 (6%) | 4 (6%) | 1.000 | |
| Arrythmia | 0 | 3 | 0.010 | 0 | 3 | 0.029 | |
| Bleeding | 5 | 0 | 4 | 0 | |||
| Endogia failure | 0 | 1 | 0 | 1 | |||
| Anatomic section | 1 | 0 | 0 | 0 | |||
| Conversion rate | 1 (1%) | 0 (0%) | 1.000 | 1 (1%) | 0 (0%) | 1.000 | |
| Mortality rate | 0 (0%) | 0 (0%) | 1.000 | 0 (0%) | 0 (0%) | 1.000 | |
| Lymphadenectomy* | |||||||
| LN (number) | 14.3±0.8 | 17.1±0.9 | 0.016 | 13.7±0.9 | 17.6±1.1 | 0.007 | |
| Stations (number) | 4.3±0.2 | 4.4±0.1 | 0.786 | 4.1±0.1 | 4.3±0.1 | 0.212 | |
| Postoperative (30 days) | |||||||
| Complication rate | 30 (30%) | 8 (8%) | <0.001 | 19 (28%) | 6 (9%) | 0.004 | |
| TM&M | |||||||
| 1 | 10 (33%) | 1 (12%) | 0.308 | 7 (37%) | 1 (17%) | 0.389 | |
| 2 | 12 (40%) | 3 (38%) | 8 (42%) | 3 (49%) | |||
| 3a | 3 (10%) | 1 (12%) | 1 (5%) | 0 (0%) | |||
| 3b | 5 (17%) | 2 (26%) | 3 (16%) | 1 (17%) | |||
| 4a | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 4b | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 5 | 0 (0%) | 1 (12%) | 0 (0%) | 1 (17%) | |||
| Opioid usage | 18 (18%) | 30 (30%) | 0.067 | 12 (18%) | 18 (26%) | 0.215 | |
| Air leak (>5 days) | 12 (12%) | 4 (4%) | 0.076 | 7 (10%) | 3 (4%) | 0.325 | |
| Chest drain (days) | 3.9±0.4 | 2.5±0.3 | 0.005 | 3.9±0.6 | 2.6±0.3 | 0.034 | |
| ICU (days) | 0.75±0.1 | 1.1±0.2 | 0.095 | 0.9±0.1 | 1.1±0.2 | 0.306 | |
| Hospitalisation (days) | 5.1±0.5 | 4.5±0.3 | 0.305 | 5.1±0.7 | 4.4±0.3 | 0.304 | |
| Reintervention rate | 5 (5%) | 3 (3%) | 0.360 | 3 (4%) | 2 (3%) | 0.340 | |
| Bleeding | 3 | 0 | 0.125 | 1 | 0 | 1.000 | |
| Air leak | 1 | 0 | 1 | 0 | |||
| Chylothorax | 1 | 1 | 1 | 1 | |||
| Fistula | 0 | 2 | 0 | 1 | |||
| Mortality rate | 0 (0%) | 1 (1%) | 1.000 | 0 (0%) | 1 (1%) | 1.000 | |
Discrete data are expressed as number with percentages: n (%); continuous data are expressed as mean ± standard deviation. Lymphadenectomy*: analyzed if histology = lung cancer. uVATS, uniportal video-assisted thoracic surgery; uRATS, uniportal robotic-assisted thoracic surgery; LN, lymph nodes; TM&M, grading of postoperative complications following the TM&M classification system; ICU, intensive care unit.
Discussion
To the best of our knowledge, this is the largest reported series on uRATS and the first study comparing the perioperative outcomes between uVATS and uRATS for anatomic lung resections. Keeping in mind that any surgical procedure requires a certain volume of cases to reach the performance plateau, we selected the initial 100 cases to overcome the learning curve of both approaches. The same surgeon performed all the procedures to minimize bias between different surgical practices and experiences. The PSM analysis ensured that results came from two comparable patient populations.
In our series, the number of anatomic segmentectomies and the proportion of complex segmentectomies were significantly higher in the uRATS group (29). However, it should be noted that a decade has passed since the beginning of uVATS and uRATS. During this time, the refinement of the diagnostic pathway and the consolidation of minimally invasive techniques have implemented the advantages of anatomical segmentectomy with favorable outcomes in terms of perioperative morbidity and mortality, disease free survival and overall survival (30,31). Zhou and colleagues (22) reported their experience with 595 anatomic segmentectomies performed by open, VATS and robotic approaches. They observed an increase in the frequency of anatomic segmentectomy (from 9.6% in 2004–2005 to 21.9% in 2018–2019) and the proportion of complex segmentectomies (from 18.5% in 2004–2005 to 37.5% in 2018–2019); robotic segmentectomies (from 0% in 2004–2005 to 43.8% in 2018–2019) and the proportion of robotic complex segmentectomies (from 18.5% in 2004–2005 to 37.5% in 2018–2019) increased as well. These results suggested that superior skills and improved visualization of the robotic platform enable deeper dissection of the lung parenchyma, precise division of the bronchi and segmental vessels and more complex segmental resections.
We observed a significantly higher number of sleeve resections performed by uRATS (32,33). It is worth mentioning that the anastomosis technique is different between the two approaches. Due to the interference with robotic arms, the threads must be short and the anastomosis should be performed in two rows using two barbed sutures (25). We believe that extensive previous uVATS experience should be an advantage when performing complex uRATS procedures, as it provides the necessary skills and confidence to solve the technical difficulties of sleeve resections (14).
Our study also demonstrated that uRATS retrieved a higher number of lymph nodes. This superiority of nodal dissection is consistent with previous publications, especially a randomized clinical trial published by Jin and colleagues (23). Sharper instruments such as the Maryland or the bipolar fenestrated could facilitate the dissection and removal of deeper lymph nodes.
Additionally, uRATS was associated with a statistically lower rate of postoperative complications and shorter duration of chest drains.
This study has several limitations. First, all procedures were performed by a single experienced surgeon. Therefore, the initial learning curve for uRATS was lost and the surgical results obtained in this study are not generalizable to daily practice. Further randomized controlled trials will be necessary to generate quality scientific evidence. Secondly, it only reports short-term perioperative outcomes. Ongoing follow-up will clarify any differences in long-term survival between uniportal robotic and thoracoscopy-assisted surgery. And last but not least, the cost associated with uRATS was not analyzed comparatively to uVATS. The higher expenses of robotic systems have been the main point of criticism, questioning their value. A recent systematic review (34) showed significant variability between institutions, with the highest cost corresponding to the initial experience. In contrast, the lowest costs came from high-volume centers with lower complication rates and shorter hospital stays. Although price should remain a consideration, it should not dictate the surgical approach. Postoperative outcomes are crucial and should ultimately determine what benefits patients.
In conclusion, our results confirm the safety, feasibility and efficacy of uRATS as a new minimally invasive technique that combines the benefits of the uniportal method and robotic systems. The convergence of these two trends has resulted in a new single-port robotic system, the da Vinci Surgical System SP®, designed for subxiphoid and subcostal thoracic approaches (35). Nowadays, it only has real value for anterior mediastinal lesions and thymectomy (36), while its use for lobectomy is still under development. We expect the integration of robotic staplers and the improvement of the SP platform to be ready in the near future. Therefore, considering the excellent results of our study, we believe that the da Vinci Surgical System Xi® is the best platform for performing robotic anatomic lung resections through a single intercostal incision.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Ng CSH, MacDonald JK, Gilbert S, et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innovations (Phila) 2019;14:90-116. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72.
- Hu J, Chen Y, Dai J, et al. Perioperative outcomes of robot-assisted vs video-assisted and traditional open thoracic surgery for lung cancer: A systematic review and network meta-analysis. Int J Med Robot 2020;16:1-14. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- McElnay PJ, Molyneux M, Krishnadas R, et al. Pain and recovery are comparable after either uniportal or multiport video-assisted thoracoscopic lobectomy: an observation study. Eur J Cardiothorac Surg 2015;47:912-5. [Crossref] [PubMed]
- Chung JH, Choi YS, Cho JH, et al. Uniportal video-assisted thoracoscopic lobectomy: an alternative to conventional thoracoscopic lobectomy in lung cancer surgery? Interact Cardiovasc Thorac Surg 2015;20:813-9. [Crossref] [PubMed]
- Zhu Y, Liang M, Wu W, et al. Preliminary results of single-port versus triple-port complete thoracoscopic lobectomy for non-small cell lung cancer. Ann Transl Med 2015;3:92. [Crossref] [PubMed]
- Liu CC, Shih CS, Pennarun N, et al. Transition from a multiport technique to a single-port technique for lung cancer surgery: is lymph node dissection inferior using the single-port technique?†. Eur J Cardiothorac Surg 2016;49:i64-72. [Crossref] [PubMed]
- Hirai K, Takeuchi S, Usuda J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: a retrospective comparative study of perioperative clinical outcomes. Eur J Cardiothorac Surg 2016;49:i37-41. [Crossref] [PubMed]
- Shen Y, Wang H, Feng M, et al. Single- versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched study†. Eur J Cardiothorac Surg 2016;49:i48-53. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49:i6-16. [Crossref] [PubMed]
- Soultanis KM, Chen Chao M, Chen J, et al. Technique and outcomes of 79 consecutive uniportal video-assisted sleeve lobectomies. Eur J Cardiothorac Surg 2019;56:876-82. [Crossref] [PubMed]
- Sekhniaidze D, Gonzalez-Rivas D, Kononets P, et al. Uniportal video-assisted thoracoscopic carinal resections: technical aspects and outcomes. Eur J Cardiothorac Surg 2020;58:i58-64. [Crossref] [PubMed]
- Van Raemdonck D, Sihoe AD. Postgraduate symposium on general thoracic surgery. Lisbon, Portugal: the European Society of Thoracic Surgeons at the 23rd European Conference on General Thoracic Surgery; 2015 May 31.
- Hansen HJ. VATS lobectomy: multiportal access is the optimal access. Lisbon, Portugal: Breakfast Session conducted by the European Society of Thoracic Surgeons at the 23rd European Conference on General Thoracic Surgery; 2015 Jun 2.
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- Kneuertz PJ, Singer E, D'Souza DM, et al. Hospital cost and clinical effectiveness of robotic-assisted versus video-assisted thoracoscopic and open lobectomy: A propensity score-weighted comparison. J Thorac Cardiovasc Surg 2019;157:2018-2026.e2. [Crossref] [PubMed]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg 2019;28:526-34. [Crossref] [PubMed]
- Zhou N, Corsini EM, Antonoff MB, et al. Robotic Surgery and Anatomic Segmentectomy: An Analysis of Trends, Patient Selection, and Outcomes. Ann Thorac Surg 2022;113:975-83. [Crossref] [PubMed]
- Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann Surg 2022;275:295-302. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Motas N, et al. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg 2022;62:ezac410. [Crossref] [PubMed]
- Gonzalez-Rivas D, Manolache V, Bosinceanu ML, et al. Uniportal pure robotic-assisted thoracic surgery—technical aspects, tips and tricks. Ann Transl Med 2022; [Crossref]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Paradela de la Morena M, De La Torre Bravos M, Fernandez Prado R, et al. Standardized surgical technique for uniportal video-assisted thoracoscopic lobectomy. Eur J Cardiothorac Surg 2020;58:i23-33. [Crossref] [PubMed]
- Rivas DG, Bosinceanu M, Dunning J. Uniportal Robotic Surgery: A Step-by-Step Guide to Setup by Diego Gonzalez Rivas. CTSNet 2022. doi:
10.25373/ctsnet.21092140.v1 - Gonzalez-Rivas D, Manolache V, Bosinceanu M, et al. Uniportal Pure Robotic-Assisted Thoracic Surgery—Left Bisegmentectomy S9-S10. CTSNet 2022. doi:
10.25373/ctsnet.20412570 - Stamatis G, Leschber G, Schwarz B, et al. Perioperative course and quality of life in a prospective randomized multicenter phase III trial, comparing standard lobectomy versus anatomical segmentectomy in patients with non-small cell lung cancer up to 2 cm, stage IA (7th edition of TNM staging system). Lung Cancer 2019;138:19-26.
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Gonzalez-Rivas D, Essa RA, Motas N, et al. Uniportal robotic-assisted thoracic surgery lung-sparing carinal sleeve resection and reconstruction. Ann Cardiothorac Surg 2022; [Crossref]
- Gonzalez-Rivas D, Prado RF, Garcia-Perez A, et al. Bilateral uniportal robotic-assisted thoracic surgery sleeve lobectomy for a bilateral endobronchial lung cancer. Ann Cardiothorac Surg 2023;12:64-6.
- Singer E, Kneuertz PJ, D'Souza DM, et al. Understanding the financial cost of robotic lobectomy: calculating the value of innovation? Ann Cardiothorac Surg 2019;8:194-201. [Crossref] [PubMed]
- Gonzalez-Rivas D, Ismail M. Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience. J Thorac Dis 2019;11:231-9. [Crossref] [PubMed]
- Park SY, Lee JH, Stein H, et al. Initial experience with and surgical outcomes of da Vinci single-port system in general thoracic surgery. J Thorac Dis 2022;14:1933-40. [Crossref] [PubMed]








