Uniportal fully robotic-assisted major pulmonary resections
Introduction
Over the past few decades, the surgical treatment of lung pathologies has progressively evolved from open surgery to minimally invasive techniques, including multiportal video-assisted thoracoscopic surgery (MVATS), uniportal video-assisted thoracoscopic surgery (UVATS) and more recently with the latest technologies, robotic-assisted thoracoscopic surgery (RATS) (1). RATS for major pulmonary resections has been proven to be safe, alongside being oncologically and economically cost-effective, thus offering patients excellent outcomes that could potentially be better in comparison to traditional approaches (2-4).
The evolution of the uniportal approach has also demonstrated rapid growth thanks to the help of the latest technology developed over the last decade (5-7). Since our group performed the first UVATS lobectomy in the world in 2010 (5), we evolved the technique to do increasingly more complex cases thanks to the acquired experience, slimmer instruments, better high-definition cameras and powered, more angulated staplers. In 2018, we were involved in the development of a novel robotic platform to adapt the technique for lobectomies and thymectomies via a subxiphoid approach by using the single port (SP®) robotic platform in a cadaveric model (8). However, several limitations were found with this system, impeding the performance of anatomic lung resections. These issues were primarily the limited access offered by the subxiphoid view, strength of the robotic arms, and the absence of robotic staplers.
In the meantime, whilst waiting for the improvements and validation of this new robotic system for major thoracic pulmonary resections, we decided to adapt the Davinci Xi® for the uniportal robotic-assisted thoracoscopic surgery (URATS) approach, performing the first pure robotic cases in the world in September 2021, in Spain (9,10). We started developing of the URATS technique by first completing several cases of biportal RATS. Since then, we have performed 150 anatomic resections including all segmentectomies, sleeves (11), and even double sleeves and carinal resections (12). Uniportal access for robotic thoracic surgery presents itself as a natural evolution of minimally invasive thoracic surgery. Robotic surgery was initially started as a hybrid procedure with the use of thoracoscopic staplers by the assistant (13). However, due to the evolution of robotic modern platforms, the staplers can be nowadays controlled by the main surgeon from the console.
The pure URATS approach is defined as robotic thoracic surgery performed through a single intercostal incision, without rib spreading, using the robotic camera, robotic dissecting instruments and robotic staplers. We always recommend the transition from multiportal robotic-assisted thoracoscopic surgery (MRATS) to URATS through biportal-RATS (14,15), doing several anatomic resections first. It is important to differentiate a pure approach from the hybrid technique (URATS-UVATS) where the surgeon only does the dissection from the console and the assistant takes over the critical part of inserting the thoracoscopic staplers through the utility incision (16). For this hybrid technique, the stapler incision should be in a similar location as for UVATS [4th or 5th intercostal space (ICS)] to allow the assistant to safely insert the staplers. We see a significant disadvantage of this hybrid technique, primarily in that it leaves the assistant to perform one of the most difficult parts of the approach for anatomic resections, inserting and firing the stapler).
Preparation and exposition
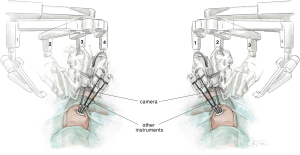
For URATS, all three arm joints should be parallel at the top, centered in the FLEX position and rotated towards the anterior plane. Conventional targeting is not necessary. The cross of the laser should be placed in the upper part of the skin incision posteriorly, parallel to the spine. To avoid collision, we must cancel arm 1 when operating on the right side of the patient (arm 2 for camera, arm 3 for the left hand and arm 4 for the right hand). When operating on the left side, we have to cancel arm 4 (arm 3 for camera, arm 1 for the left hand and arm 2 for the right hand). The camera is normally placed in the posterior part of the incision to allow the other two robotic instruments working below to be parallel, along the sagittal plane (see Figure 1) (9). The use of the TORS trocars (used for transoral surgery) further improves the freedom of movement for URATS, particularly if the additional lateral piece is removed (not needed for thoracic surgery, only necessary when working through the oral cavity). Video-assisted thoracoscopic surgery (VATS) instruments and an open surgery set must always be in the operating room during URATS cases in the instance of emergencies. The use of two long curved Dennis Suction devices (Scanlan international) is very helpful in obtaining better exposure with the help of the assistant. This suction device must be inserted inferior to the trocar used for the camera to minimize interference with the robotic instruments. The robotic instruments mostly used in URATS are bipolar fenestrated on left hand for grasping tissue and Maryland Bipolar Forceps for dissection. For specific maneuvers, we can use the Tip-Up Fenestrated Grasper in the case of encountering a large artery or vein. A monopolar hook or spatula for adhesions and scissors and needle holders for sleeve resections are also convenient. Monopolar instruments allow for a quick dissection but generate thermal spread which might be harmful to adjacent structures. Bipolar instruments enable a precise dissection around structures such as the pulmonary artery sheath, parabronchial tissue and phrenic or vagus nerve. However, the dissection is slower, and the jaws need to be slightly open to grasp and cauterize tissue in a correct, safe manner. We should be careful with the base of the Maryland when using coagulation to avoid collateral damage.
In case of small vessels, the use of polymer clips is an option (Click aV plus, Grena Ltd., Brentford, UK) (see Figure 2). We normally recommend the use of two proximal clips and energy for the distal part of the vessel with vessel sealer or synchroseal. Compared to UVATS, the use of robotic staplers is easier than in URATS as the incision is lower and the bigger angulation inside allows for an easier insertion of the robotic staplers. We always recommend using a 45-stapler, and the curved tip (Sureform, Intuitive Surgical, Sunnyvale, CA, USA) is very helpful in achieving a good angle particularly for upper lobes. The use of 60 staplers is not convenient because its angulation internally is restricted. One of the reasons for placing an incision in an inferior location is to allow for this correct articulation of the staplers internally. It is very important to coordinate and place arms in their correct order to prevent loss of internal articulation, as in the instance when the arms are crossed. In some steps, the staplers should be used from the left hand to achieve good angles. The camera should be at 30 degrees and normally with a downwards orientation. However, in case of adhesions, it could be necessary to switch the angulation of the camera, with 30° upwards, working with the instruments above the camera. Changing the angulation of the 30° camera and adjusting the arm placement according to upper or lower locations, allows us to reach every cm inside the chest cavity with no restrictions.
Surgical technique
Here we describe the surgical technique for right upper and left upper lobectomy.
Right upper lobectomy
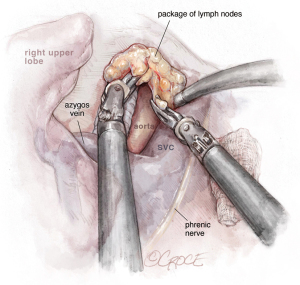
The incision should be placed in the 7th ICS, between the anterior and middle axillary lines. After initial evaluation, the dissection is performed and the hilum-first approach is recommended, from anterior to posterior, with artery-vein-bronchus sequencing or alternatively, vein-artery-bronchus, depending on anatomy particularities and surgeon preference. In URATS, we complete vein first (see Figure 3) and then artery (anterior trunk) (see Figure 4), as it seems more natural given the angle for stapling the vein is not as difficult as in UVATS. A vessel loop is always very helpful to allow for a safe and easy insertion of the staplers (17). We usually insert a cigar made with a gauze at the beginning of the operation to facilitate retraction of the lobe and exposure of the hilum, which is done by the main surgeon at the console. During dissection, grasping and mobilizing the upper lobe is much better handled with the robotic fenestrated bipolar grasper than with a VATS grasper, in order to avoid collision between the arms; the optimal exposure is facilitated by the use of the long-curved Dennis suction inserted by the assistant, without the need for grasping the lung, again to avoid collision. For identification of the right upper lobe (RUL) bronchus, it is helpful to remove the lymph nodes that are normally present at its base first. Rotation of the lobe facilitates the posterior dissection before starting the anterior dissection. The use of long curved tip up dissector is very useful for the bronchus (see Figure 5). Once dissected, we also recommend traction with a vessel loop held by the bipolar fenestrated on the left hand. The stapler is normally inserted with the right hand with angulation, anvil down, orientated to the left. The posterior ascending artery is then dissected (see Figure 6) and divided by using polymer clips (Click AV plus, Grena Ltd.) or a robotic stapler.
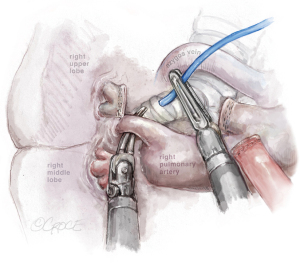
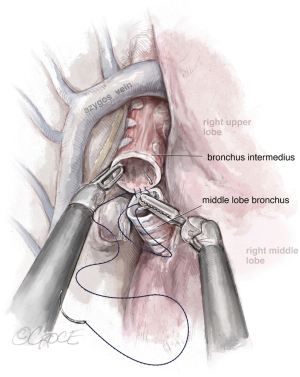
The last step is the division of the anterior part of the fissure between the upper and middle lobe (see Figure 7) where it is important to identify the middle lobe artery and to free the upper lobe vein venous stump. The stapler is placed between them. The fissure-last technique became our usual approach in URATS right upper lobectomies. For lung cancer patients, a systematic mediastinal lymph node dissection must be performed. The technique is different when compared to UVATS, with the dissection normally being performed with the bipolar fenestrated and the Maryland. However, in case of radical lymphadenectomy, we recommend the use of a vessel sealer or synchroseal in order to ensure a proper sealing of vessels and to avoid chylothorax. For dissection of the subcarinal station, it is better to retract the lung with the use of two long curved suctions by the assistant, enabling the surgeon at the console to advance with the dissection.
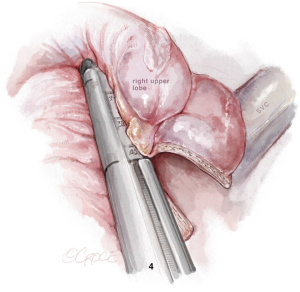
Left upper lobectomy
The incision is placed in the 7th ICS, between the anterior and middle axillary lines. The technique is similar to that on the right side. Because the incision is more lateral and more inferior, when the instruments are inserted into the chest cavity, the first thing we encounter is the fissure. If the fissure is open, we prefer to start the surgery by dissecting the lingular artery (see Figure 8) and posterior ascending branches from the fissure [for left upper lobe (LUL) there could be many variations in the number of arteries]. We can divide the anterior or posterior part of the fissure when present, with robotic staplers (see Figure 9).
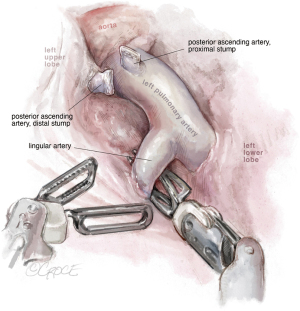
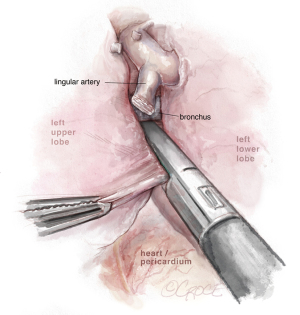
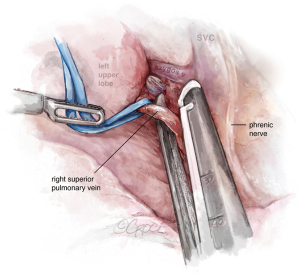
The next step should be the vein dissection (see Figure 10). It is important to identify both the upper and lower veins in order to avoid a mistake by stapling a common trunk, which would end up in an unexpected left pneumonectomy. We recommend removing first the lymph nodes from stations 10, 5 and 6. After dissecting the upper vein, we use a vessel loop to keep traction of the vessel allowing an easier insertion of the robotic angulated stapler. The next step would be to dissect and divide the anterior and apico-posterior branches that is facilitated if the posterior ascending artery was divided from the fissure.
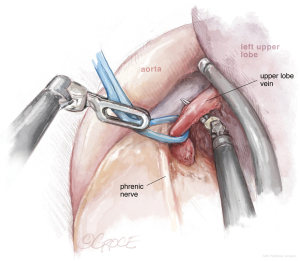
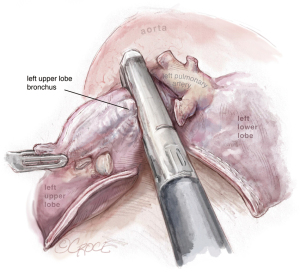
Dissection of the bronchus must be carefully done, because on its posterior part lies the pulmonary artery. The URATS offers a more inferior view and somehow facilitates the dissection in this moment, if the exposure is correctly done. The stapler for the bronchus is inserted while protecting the interlobar pulmonary artery and it’s not fired before checking, by inflating the lower lobe (see Figure 11). If the fissure is absent, we perform a fissure-less lobectomy in the following order: vein, anterior and apical artery, posterior artery, LUL bronchus, lingular artery and fissure last. If necessary, lymph node dissection in the subcarinal stations 8 and 9 is performed, as well as station 7, from a posterior approach, as we do in UVATS.
Complex resections
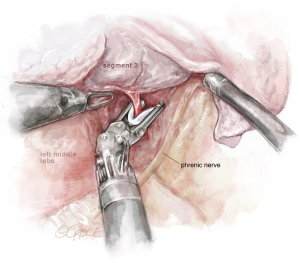
The URATS technique is very suitable for anatomic segmental resections, especially for the segments located more posteriorly, as the robot provides more maneuverability and deeper 3-dimensional views compared with UVATS. The characteristics of some of the sharper instruments used with the robot, such as the Maryland device, facilitate the dissection for distal segments and subsegments and also the removal of deeper intrapulmonary lymph nodes (18). The URATS approach is also feasible for more advanced resections. For sleeve procedures, we use the camera on the 2nd arm, Maryland on the left hand (3rd arm) and the needle holder on the right hand (4th arm). On the left side, we recommend placing the camera on the 3rd arm, Maryland on the left arm (2nd) and the needle holder on the right hand (1st arm). For the bronchial anastomosis, the best strategy is to use a barbed suture, and we recommend performing a running suture, one thread of 25 or 30 cm (depending size of bronchus) with two needles, 17 mm in size for both needles (see Figure 12). This type of suture is more reliable and safer in RATS because tension of the thread is not lost while doing the anastomosis (maintaining the tension by the assistant during the procedure is difficult) (11).
The other option is to perform the anastomosis using two barbed sutures, one for the posterior wall and the other for the anterior wall. When pneumonectomy is needed, we recommend the use of a tool called “fenestrated Tip up”, which is longer and facilitates the dissection of the main pulmonary artery and retraction of the bronchus. We consider the bronchial division for pneumonectomy by URATS as opposed to UVATS, because the insertion of the stapler via the 6th or 7th ICS helps to achieve a shorter stump of the left main bronchus (19).
Completion
When surgery is completed, we always block the ICSs (3–4 spaces with levobupivacaine 0.25%), check for hemostasis and possible air leak. Finally, a chest tube (normally 24 French) is placed. The positioning of the tube is done meticulously, placing the chest tube at the upper part of incision in order to avoid infection or pulmonary herniation.
Comments
Clinical results
Since September 2021, our surgical team has performed 150 cases of URATS resections across 12 countries internationally. A study on the learning curve comparing the results of the first 100 URATS cases (all performed by the same surgeon) versus the learning curve of the first UVATS cases (surgeries performed from 2010) was conducted. The proportion of URATS resections were as follows: 32% anatomic segmentectomies, 58% lobectomies, 5% bilobectomies, 3% pneumonectomies and 2% tracheal and carinal resections. Regarding surgical complications, the intraoperative bleeding was more frequent in the UVATS group, and arrhythmia in the URATS group. Postoperative complication rate was higher in the UVATS than in URATS (28% vs. 9%, P=0.004). Both groups had no significant difference regarding opioid usage and prolonged air leak. Duration of chest drain insertion was significantly higher in the UVATS than in URATS (3.9 vs. 2.6 days, P=0.034). Length of intensive care unit (ICU) and hospitalization, reintervention rate and mortality rate were similar in both groups (19). We also analyzed our URATS outcomes performed in several European centers by a single surgeon (101 cases), compared to another 101 MRATS cases from a different team in Spain. The median operative time was statistically lower in URATS group (136 vs. 150 min) and the median length of stay was significantly shorter in the URATS group (4 vs. 5 days). Rate of complications and 30-day mortality was low in both groups (20).
To date, a total number of 30 URATS sleeve resections have been performed by the same surgeon. The mean surgical time was 178±5.5 minutes, and the mean hospital length of stay was 6.6±0.5 days. One patient with critical comorbidities died during the first 30 postoperative days due to acute respiratory distress syndrome (ARDS) (11).
Advantages and caveats
URATS offers the advantage of robotic surgery (i.e., instruments and staplers through a single anterior incision) and the potential result for less pain and better postoperative outcomes as previously described in UVATS (5). The docking time and port placement is quicker, with no need for targeting. As we only use three arms (two instruments instead of three) and no air seal is needed, the procedure is cheaper compared to MRATS. The high cost of the robotic platform is one of the main limitations for the introduction in many centers around the world. Additionally, it is not an easy technique to learn and the role of the assistant is very important, more so than in MRATS or UVATS. The intraoperative instrumentation in URATS is different compared to MRATS, so there is a new learning curve for those experienced robotic surgeons when learning how to avoid collision of the instruments (21). The assistant surgeon must be skilled and familiarized with the UVATS technique. Once these new movements are mastered and the correct technique is learned, the collision never happens in experienced URATS surgeons. Although docking is quicker, the time during lobectomy could be increased due to the assistant replacing one of the 8 mm trocars with one 12 mm, for the stapler insertion and readjustment of the arms each time. In order to save time, it is recommended to remove the staplers en-block with the trocars after the stapling is done. By doing this we speed up the procedure and we prevent the block of the system that sometimes happens when the stapler is not correctly aligned before removal.
One of the main difficulties for pure URATS is when the chest cavity is small. The insertion and angulation of the staplers could be compromised by the limited space. If the surgeon at the console does not feel in complete control of the robotic staplers, we recommend the use of thoracoscopic staplers, and never to force the manipulation of the staplers inside the thorax. The 8 mm staplers facilitate these movements, allowing a better control from the console.
We recommend the transition from MRATS to URATS doing a few cases with biportal RATS. For biportal RATS, we recommend placing the utility incision in the 5th ICS and the additional incision in the 7th ICS. The camera must be placed in the upper part of the incision as in UVATS and the robotic instrumentation is performed with triangulation of the instruments, placed in the lower part of the incision and through the accessory port. It is recommended that the surgeon becomes confident with this biportal robotic approach before moving to URATS. Table 1 summarizes the advantages and caveats in URATS.
Table 1
| Advantages of URATS | Caveats of URATS |
|---|---|
| Cheaper (only 3 arms, no CO2, no guide, no adaptor for CO2, no need of air seal) | More demanding technique: assistant is responsible for 50% of the surgery |
| Rapid docking, no need for targeting (1 min) | Longer learning curve |
| Less painful (single incision anterior approach) | Time consuming compared to UVATS (frequent readaptation of arms, clearance, change trocar for staplers) |
| Easy to undock in case of bleeding | A set of long thoracoscopic instruments is needed (Scanlan international) |
| Faster removal of staplers (en-bloc with trocar) | Utility incision is lower than UVATS (more difficult for assistant) |
| Faster conversion to thoracotomy (enlarge the incision) | In case of hybrid technique, assistant must be familiarized with UVATS technique |
| Easier than MRATS in case of strong adhesions (docking is difficult for MRATS in case of aspergilloma cases or TBC) | |
| Makes non-intubated technique feasible | |
| 8 mm staplers will make it easier |
URATS, uniportal robotic-assisted thoracoscopic surgery; MRATS, multiportal robotic-assisted thoracoscopic surgery; TBC, tuberculosis; UVATS, uniportal video-assisted thoracoscopic surgery.
Future of uniportal and robotic surgery
The evolution of surgery must follow the principle of the least invasive approach whilst adopting the use of the most effective and precise technology. With this in mind, we are sure that the uniportal robotic approach will generate more interest among thoracic surgeons and become more popular in the coming years. Since it is now proven that the existing Da Vinci Xi platform is suitable for all kinds of intrathoracic resections with excellent outcomes, the possibilities of improvement are promising. The design of specific, thinner trocars, narrow instruments and 8 mm staplers will help the technique to be easily adopted worldwide. In the last few years, there has been a convergence of the uniportal approach and robotic-assisted surgery which resulted in a pure, single port robotic system; the Da Vinci SP by Intuitive Surgical (Sunnyvale, California, USA). The SP platform is notable for a single 2.5 cm cannula through which an articulating 3-dimensionnal camera and three fully articulating instruments with 7 degrees of freedom can be passed. With its commercial introduction, our group reported the first robotic uniportal lobectomies with SP by conducting experimental cadaver lab dissections in the research setting in 2018. However, staplers for this new platform were not yet developed, forcing the system to be hybrid and not easy for lobectomies where the assistant must introduce the thoracoscopic staplers via a subxiphoid access.
As robotic development grows, we believe the indications for SP robotic lobectomies will be extended to include an increasing number of procedures including increasingly more complex resections. In the future, the implementation of robotic staplers in the SP platform will allow the surgeon at the console to perform the whole anatomic resection in a fully robotic setting, which may increase the efficiency and safety of the operation. However, this development will take several years to be ready and reliable for thoracic surgeons, who are not normally familiarized with a subxiphoid approach. At this moment of writing, we can only say that from our experience, the SP platform is a good tool for thymectomies (22). For this reason, a good alternative to the SP is to learn the URATS technique with the available Da Vinci Xi system, using robotic staplers for a fully robotic approach.
Conclusions
URATS is here to stay, offering huge potential in the future of thoracic surgery with the development of slimmer and more precise instruments, better imaging and the specific SP platform. The single incision combined with the robotic technology offers the least invasive approach for patients with the most precise surgical instrumentation, alongside excellent outcomes.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Yang S, Guo W, Chen X, et al. Early outcomes of robotic versus uniportal video-assisted thoracic surgery for lung cancer: a propensity score-matched study. Eur J Cardiothorac Surg 2018;53:348-52. [Crossref] [PubMed]
- Veronesi G. Robotic thoracic surgery: technical considerations and learning curve for pulmonary resection. Thorac Surg Clin 2014;24:135-41. v. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- González-Rivas D, Garcia A, Chen C, et al. Technical aspects of uniportal video-assisted thoracoscopic sleeve resections: Where are the limits? JTCVS Tech 2020;2:160-4. [Crossref] [PubMed]
- Gonzalez-Rivas D, Garcia A, Chen C, et al. Technical aspects of uniportal video-assisted thoracoscopic double sleeve bronchovascular resections. Eur J Cardiothorac Surg 2020;58:i14-22. [Crossref] [PubMed]
- Gonzalez-Rivas D, Ismail M. Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience. J Thorac Dis 2019;11:231-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Manolache V, Bosinceanu ML, et al. Uniportal pure robotic-assisted thoracic surgery—technical aspects, tips and tricks. Ann Transl Med 2022; [Crossref]
- Gonzalez-Rivas D, Bosinceanu M, Motas N, et al. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg 2022;62:ezac410. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Manolache V, et al. Uniportal fully robotic-assisted sleeve resections: surgical technique and initial experience of 30 cases. Ann Cardiothorac Surg 2023;12:9-22.
- Gonzalez-Rivas D, Essa RA, Motas N, et al. Uniportal robotic-assisted thoracic surgery lung-sparing carinal sleeve resection and reconstruction. Ann Cardiothorac Surg 2022; [Crossref]
- Yang Y, Song L, Huang J, et al. A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) Surgical System in the treatment of early-stage lung cancer. Transl Lung Cancer Res 2021;10:1571-5. [Crossref] [PubMed]
- Yang N, He X, Bai Q, et al. Analysis of the short-term outcomes of biportal robot-assisted lobectomy. Int J Med Robot 2021;17:e2326. Erratum in: Int J Med Robot 2022;18:e2436. [Crossref] [PubMed]
- Qu JC, Zhang WT, Jiang L. Two-port robotic sleeve lobectomy using Stratafix sutures for central lung tumors. Thorac Cancer 2022;13:1457-62. [Crossref] [PubMed]
- E H. Hybrid uniportal robotic-assisted thoracoscopic surgery (RATS) using video-assisted thoracoscopic surgery (VATS) staplers: technical aspects and results. Ann Cardiothorac Surg 2023;12:34-40.
- Dunning J, Waterhouse B, Rivas DG. The UK’s First Uniportal Robotic Surgery with Diego Gonzales Rivas. 2022. doi:
10.25373/ctsnet.21632624.v1 . Available online: https://www.ctsnet.org/article/uks-first-uniportal-roboticsurgery-diego-gonzales-rivas - Motas N, Manolache V, Bosinceanu ML, et al. Uniportal roboticassisted thoracic surgery anatomic segmentectomies. Ann Cardiothorac Surg 2022; [Crossref]
- Motas N, Gonzalez-Rivas D, Bosinceanu ML, et al. Uniportal robotic-assisted thoracic surgery pneumonectomy. Ann Cardiothorac Surg 2023;12:67-9.
- Paradela M, Garcia-Perez A, Fernandez-Prado R, et al. Uniportal robotic versus thoracoscopic assisted surgery: a propensity score-matched analysis of the initial 100 cases. Ann Cardiothorac Surg 2023;12:23-33.
- Manolache M, Motas N, Bosinceanu M, et al. Comparison of Uniportal RATS anatomic resections with MRATS-multicenter study: the European experience. Ann Cardiothorac Surg 2023; [Crossref]
- Park SY, Lee JH, Stein H, et al. Initial experience with and surgical outcomes of da Vinci single-port system in general thoracic surgery. J Thorac Dis 2022;14:1933-40. [Crossref] [PubMed]