Aortic valve-sparing root replacement or Bentall?
Introduction
The modern era of aortic root surgery is informed by multiple significant advancements since Bentall and De Bono first described their use of a mechanical valved conduit for replacement of the aortic valve and ascending aorta in 1968 (1). Aortic root replacement was developed to treat diseases of the ascending aorta and aortic sinuses most often caused by aneurysmal degeneration. For many years, the Bentall operation, with or without modification, was the only surgical solution, but was marked, even in experienced hands, by perioperative mortality of approximately 10% whether mechanical or bioprosthetic valves were used (2,3). Bentall and De Bono’s original description highlighted an aorta that was dilated and thinned down all the way to the annulus, precluding the usual approach of joining the aortic prosthesis to the aortic wall just above the coronaries. They sutured a tube prosthesis directly to the ring of a Starr valve, which was then tied down to sutures in the aortic annulus. Holes were then cut in the aortic prosthesis at the level of the coronary ostia and the aortic wall was sutured to the perimeter of the holes to reincorporate the coronary ostia. The distal anastomosis was then completed, and the wall of the aneurysm closed over the prosthesis (1).
Many modifications were described over the next decades, most notably the conversion from the inclusion to exclusion technique of excising the aneurysmal aorta to create coronary buttons—an aortic cuff around the coronary ostia—allowing for extensive mobilization and decreased tension on the coronary ostia anastomoses. This resulted in significant reductions in both pseudoaneurysm formation at suture lines and the frequency of reoperations on the ascending aorta (3,4). Additional modifications to the original Bentall procedure have included pretreating the aortic prosthesis to improve hemostasis by decreasing porosity, various strategies for challenging coronary artery reattachments including that described by Cabrol utilizing smaller Dacron interposition grafts (5), expansion to include the use of bioprosthetic valves, pre-assembled valved conduits, and aortic grafts with neo-sinuses of Valsalva (6).
The introduction of the exclusion technique and other refinements in surgical strategy and critical care dramatically improved the outcomes for patients in need of aortic root replacement. Contemporary series report an operative mortality for the modified Bentall procedure in experienced aortic centers of less than 2%, with a marked decline in the incidence of stroke, hemorrhage and other major postoperative complications compared to earlier decades (7-9). Although the use of a mechanical valve continues to be accompanied by significant lifetime risk of both thromboembolism and major hemorrhagic complications (10), improved durability of bioprosthetic aortic valves and potential percutaneous options for subsequent valve replacement have altered the expectations of many patients with disease of the aortic root, including young patients who wish to avoid anticoagulation. Valve-sparing root reimplantation (VSRR), first described by David and Feindel in 1991, introduced yet another modification to surgical technique that allowed patients with appropriate anatomy to retain their native aortic valve during aortic root replacement (11). Many of the diseases leading to aortic root aneurysms, such as Marfan syndrome, bicuspid aortopathy, Loeys-Dietz syndrome, other connective tissue disorders and undefined aortopathies largely result in degeneration of the aortic tissue whilst sparing the aortic valve leaflet tissue. Regurgitation or dysfunction of the aortic valve may be due to annulo-aortic ectasia, or dilation of the sinotubular junction (STJ). As a result, many patients with aortic root disease may still have relatively normal aortic valve leaflets. This realization opened the door for development of valve-preserving root replacement techniques.
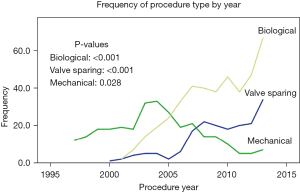
VSRR using the reimplantation technique involves resecting the aneurysmal aortic tissue and reimplanting the entire aortic valve apparatus inside a tubular Dacron graft (Figure 1) (11). With proper sizing of the graft diameter, the reimplantation procedure restores proper geometry to the valve, annulus and STJ, thus allowing the valve to function properly. Performing VSRR with a normal, well-functioning trileaflet aortic valve without aortic insufficiency (AI) leads to the most predictably durable results, and the technique has been applied in a variety of clinical presentations and valvular abnormalities with reasonable success. By preserving the native valve leaflets, the valve has the potential to last a lifetime, avoiding the inevitable structural degeneration associated with bioprosthetic valves or need for lifelong anticoagulation with mechanical valves. The numerous developments in the field of aortic root surgery contributed heavily to the increase in the number of aortic root replacement procedures performed in the last two decades. Data from the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database revealed a linear increase in proximal aortic surgery in the United States between 2004 and 2009, with aortic root operations representing approximately 5% of all cardiac procedures performed (8,13,14). Along with the increase in number of conduit valved graft (CVG) root replacements, we have seen a progressive shift away from the traditional mechanical conduit valved graft (mCVG) to more widespread adoption of the bioprosthetic conduit valved graft (bCVG) and VSRR in more recent years (Figure 2). Initial concerns regarding the safety and durability of VSRR have been assuaged by series reporting success even in higher risk patient cohorts resulting in expansion of the candidate population for VSRR and a shift toward valve preservation during root replacement.
VSRR operative technique
Our operative technique has evolved over time. In our earlier experience, the aortic valve was reimplanted into a straight tube graft. We have since adopted a sinus of Valsalva graft that aids in teaching the procedure to junior faculty and trainees. The additional space in the preformed sinus segment, a predefined STJ and pliable fabric allows for a more reproducible result and improved visualization. After cardioplegic arrest is established, the ascending aorta is resected down to the STJ. Initial assessment of the valve leaflets’ suitability for preservation is performed. Ideal leaflets are thin and pliable without major calcification, fenestrations or perforations that can portend greater risk of early structural degeneration. The aorta is resected to within 3 to 4 mm of the annulus. Coronary buttons are cut from the surrounding tissues. A plane is developed circumferentially around the aortic annulus down to the level of the left ventricular outflow tract. The ligament between the aorta and pulmonary artery at the left-right commissure may be carefully taken down, although some groups prefer to create a slit in the prosthetic graft to allow space for the ligament. A Hegar dilator is used to size the annulus. Twelve non-pledgeted sutures are placed through the left ventricular outflow tract below the level of the annulus starting at the commissures and then evenly spaced between the commissures. A graft 3–5 mm larger than the Hegar dilator sizer, up to a maximum of 32 mm, is selected. Patients with annular diameter larger than this are downsized to a 32-mm graft. The height of the commissure is measured from the aorto-mitral curtain to the top of the left non-commissure. The height of the commissure is marked on the graft from the neo-STJ towards the annulus. The excess length on the skirt of the Valsalva graft is trimmed. The valve sutures are passed through the graft at the neo-annular level and then tied down with the Hegar dilator in place to prevent an inadvertent annuloplasty effect. The commissures are lifted vertically to their natural angular configuration and resuspended at the neo-STJ. The valve is then reimplanted with running 4-0 polypropylene suture, and the left main coronary button is reattached. At this point, the valve cusps are visually assessed for prolapse or excessive free edge length. The majority of AI is improved by correcting the root dilatation with a minority of patients requiring actual cusp modification. Cusp repair, most often using central plication, is performed as needed to achieve equal free edge length and proper coaptation. Although many groups prefer to repair the cusps prior to reimplantation within the graft, we prefer to have the commissural orientation established prior to evaluating the cusps for repair. The graft is then pressurized with cardioplegia to assess left coronary perfusion using a temperature monitor in the myocardium and monitor for AI. The distal anastomosis and then right coronary button reimplantation are subsequently performed.
Early outcomes
Contemporary operative outcomes associated with both CVG and VSRR are generally excellent. In most earlier series, the majority of VSRR patients were younger with fewer comorbidities than those undergoing CVG, making comparison of root replacement strategies challenging due to selection bias. More recently, the use of VSRR has expanded to higher risk cohorts, such as those with severe AI, reoperations or type A dissections. Propensity-matched analyses have offered additional insight. In our analysis of 890 consecutive aortic root replacement procedures, we found zero in-hospital or early mortality in patients undergoing VSRR, with an overall operative mortality of 0.2% and an incidence of major postoperative complications of 0.5% (8). Surgical technique (mCVG vs. bCVG vs. VSRR) did not affect in-hospital mortality or long-term survival. In a more recent review of VSRR, we again found zero in-hospital or early mortality in patients undergoing VSRR (11). Other centers have demonstrated similarly good results. Svensson and colleagues reported on 100 propensity-matched pairs of patients with trileaflet valves undergoing elective aortic root replacement with either VSRR or CVG (15). Overall operative mortality was 0.16% and major adverse events were rare and similar between the matched groups, with the exception of pacemaker implantation occurring in greater numbers in the CVG group (6% vs. 2%, P=0.001). In a recent update from David et al., operative mortality for VSRR was 1.1%, mostly early in the experience, and other adverse events were infrequent (16). Other groups have reported VSRR operative mortality of 1.8–4%, although many of the mortalities had operations on a non-elective basis or for expanded indications, such as acute type A aortic dissection (17-19). In experienced centers, both CVG and VSRR can be performed with minimal risk of mortality or complications, especially in the elective setting. With encouraging early operative results, VSRR in various clinical settings have been assessed in detail.
VSRR with severe AI
With aortic root aneurysms, AI may be caused by abnormalities of the aortic root or of the valve leaflets themselves. Such causes include annular dilatation, sinus of Valsalva dilatation, sinotubular junction dilatation, leaflet prolapse, leaflet perforations or other valve cusp anomalies. Concerns regarding AI in the setting of VSRR are 2-fold: firstly, the AI needs to be eliminated and secondly, risk of recurrent AI requiring late reoperation must be assessed. AI associated with root dilatation may be associated with relatively preserved cusp geometry. This type of AI usually has preserved symmetry and a centrally directed AI jet. In our experience, increased aortic symmetry is associated with a reduced risk of recurrent AI on follow-up (20). AI related to a leaflet abnormality requires correction with cusp repair, which adds additional complexity and may increase risk of late recurrent AI (21).
Despite many patients starting with significant AI, careful selection and use of durable repair techniques has led to encouraging results. Kallenbach et al. reported on 158 patients undergoing VSRR, 83 of whom had 3+ or greater AI. Although the mean grade of immediate postoperative AI was higher in the group with preoperative AI (0.31±0.46 vs. 0.18±0.42, P=0.049), the difference was comparable on follow-up exams with no difference in need for reoperation (3.8% vs. 4.4%) (22). Similar results were reported by de Kerchove et al. who reported on 57% of 164 patients having 3+ or greater AI, 57% of whom needed cusp repair, having similar freedom from reoperation at eight years (89%±11% vs. 90%±7%, P=0.7) and freedom from recurrent 3+ or greater AI at five years (90%±10% vs. 89%±8%, P=0.9) (23). Their series was later updated and demonstrated freedom from reoperation of 89.6%±2.9% at ten years, while the percentage of patients with AI grade 1+ increased from 40% to 51%, 2+ from 5% to 11%, 3+ from 0% to 2% and 4+ from 0% to 1% at ten years (24). McCarthy et al. found that long-term survival was impaired in those with preoperative severe AI (HR =2.6, P=0.04) (25). Deas et al. compared eccentric AI jets to concentric jets and found no statistical difference in the cumulative risk of aortic valve replacement at ten years but the absolute numbers, 0% vs. 9.4%, may indicate further study in this area (26). In summary, VSRR may be performed in patients with preoperative moderate or severe AI, although many may require cusp interventions. Acceptable operative and long-term outcomes are achievable with careful patient selection.
VSRR with large aneurysms
Due to the aortic valve attachments along the annulus and commissures, extensive dilatation of the aortic root may create significant mechanical stress on the leaflets leading to substantial stretching of the cusps. This may cause elongation and thinning of the aortic valve cusps and fenestrations may form, especially near the commissures. The damage created by these stresses has the potential to compromise the durability of the reimplanted aortic valve. Additionally, correction of aortic root diameter may lead to leaflet prolapse if length of the free edge is severely elongated. However, many valves within large aortic root aneurysms may still be successfully preserved. Leyh et al. compared mid-term outcomes of 123 patients with aneurysms either exceeding 60, 50–60 or less than 50 mm. They found that three year survival, freedom from reoperation (98%±2% vs. 96%±3% vs. 88%±8%) and freedom from moderate or severe AI (100%±0% vs. 88%±8% vs. 94%±5%) were not significantly different between the three groups (27). Kari et al. examined a group of patients with both large aneurysms greater than 55 mm and 3+ or greater AI and found that larger aneurysms did not necessitate an increased incidence of cusp repair compared to smaller aneurysms. Freedom from early failure, valve replacement and AI 2+ or greater was 96% (28). With careful selection of adequate cusp quality, the preoperative aortic root diameter does not appear to have a significant impact on valve durability.
VSRR with bicuspid aortic valve
Valve sparing root reimplantation was initially developed and utilized for trileaflet aortic valves (TAV). As experience increased, the technique was later applied to bicuspid aortic valves (BAV). Unlike TAV, which lay in a naturally symmetric 120-120-120 degree configuration, BAVs are comprised of a heterogenous range of morphologic configurations of the commissures, cusps and sinuses. Cusp repair is more likely to be necessary with BAV compared to TAV, but operative results remain good (29). Early concerns about the operative risks and functional outcomes of reimplanting a BAV have been allayed (30,31). In the current era, VSRR with BAV has been studied extensively.
VSRR has also been compared to subcommissural annuloplasty (SCA) for BAV repair. Annular ectasia is common among BAV patients. Annular downsizing and stabilization has become an integral part of BAV repair. Among 161 patients undergoing BAV repair with VSRR or SCA, de Kerchove et al. found that residual AI was similar between groups, but peak gradient was higher among the SCA group. Freedom from reoperation (100% vs. 90%±8%, P=0.03) and freedom from greater than 2+ AI (100% vs. 77%±14%, P=0.002) were both better in the VSRR group (32). Habertheuer et al. performed a similar comparison and found no difference in reoperation up to six years but five year risk of recurrent moderate or greater AI was 15.1–15.3% for VSRR and 25–42.7% for SCA, depending on annular size of less than or greater than 30 mm (33). Compared to SCA, VSRR may provide improved durability due to enhanced annular support.
Mid-term outcomes of VSRR with BAV have been encouraging. Our group found that no patients required reoperation at five years and freedom from greater than mild AI was 92.1% vs. 100% depending on whether cusp repair was required (14). Kari et al. had similar mid-term outcomes with 90%±5% freedom from reoperation at six years and freedom from AI greater than 2+ of 100% (34). When compared to VSRR with TAV, the results of VSRR with BAV are similar. A majority of groups have found no significant difference in freedom from aortic valve reoperation or freedom from moderate or greater AI at five years (35,36). However, a consistent trend is apparent among multiple series wherein the absolute values for freedom from reintervention and freedom from moderate or greater AI are worse in the BAV group, but not statistically significant. For example, Kayatta et al. found that five year cumulative incidence for AVR was 7.7% in BAV vs. 4.3% in TAV (P=0.81) and for greater than 2+ AI, incidence was 7.7% in BAV vs 2% in TAV (P=0.75) (37). Similarly, Mokashi et al. found severe AI in 7.4% of BAV vs. 2.9% of TAV (P=0.7) and freedom from AVR was 94% in BAV vs. 98% in TAV (P=0.1) (38). Given the relatively small numbers of BAV patients, it is unclear whether these numbers are meaningful. With significantly longer follow-up out to twenty years, Klotz et al. performed a landmark analysis showing that in the second decade, cumulative incidence of reoperation was greater in BAV patients (P<0.001) (39). Beckmann et al. and Ouzounian et al. both found no difference in freedom from reintervention out to twenty years between BAV and TAV and both attributed this, at least partially, to very strict and careful patient selection (29,40). As demonstrated above, interpretation of the literature on VSRR with BAV is difficult due to the variable anatomic morphology of BAVs, differing repair techniques, variable length and quality of follow-up and differences in patient selection criteria among surgeons. Clearly, the community may benefit from larger studies with longer follow-up but at least in well-selected BAV patients, VSRR is a reasonable option.
VSRR with aortic valve cusp repair
Cusp repair for the correction of AI during VSRR is often required when the valve does not have perfect symmetry. One or more cusps may prolapse, leading to AI. Correction of this issue requires a balance between under-correction, resulting in persistent prolapse, and over-correction, resulting in cusp restriction and stenosis. Bicuspid aortic valves are more likely to require cusp repair than TAV due to a higher incidence of asymmetry and prolapse between the fused and flat leaflets, especially with Sievers I BAVs. Many types of repair have been attempted with varying success, including central plication, commissural plication, free margin resuspension, triangular resection and patch-plasty. In general, simpler repairs such as central plication (Figure 3) hold up well while complex repairs such as patching may have a higher risk of failure. Kari et al. found that free margin shortening was durable whilst commissural suspension was associated with recurrent AI (34). Meanwhile, David et al. found that free margin reinforcement was associated with late moderate or severe AI whilst cusp plication was not (41).
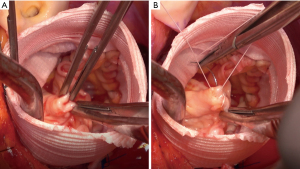
Some groups have noted worse durability when cusp repair is required, especially with BAV. A multi-institutional study by De Paulis et al. found that the incidence of reoperation at ten years was significantly greater among patients who had cusp repair (HR =1.67; P=0.004) (21). Similarly, Settepani et al. noted that cusp repair did not affect risk of reoperation with TAV, but BAV requiring cusp repair had significantly increased risk of reoperation (94% vs. 58% freedom from reoperation, P=0.04) (42).
Other groups take a more liberal approach to cusp repair with good mid- to long-term durability. Bavaria et al. were aggressive in repairing 100% of BAV vs. 6% of TAV, mostly with central plication (90%) and achieved five year freedom from reoperation of 100% vs. 97% and only 3/129 patients had moderate or greater AI (35). Liebrich et al. also repaired a majority of cusps with central plication with a minority receiving free margin reinforcement, commissural resuspension, commissural narrowing or pericardial patch augmentation. The ten year freedom from reoperation was 92% without cusp repair and 89% with cusp repair (P=1) (43). Preoperative valve function may affect recurrence of AI. In a study by Tamer et al., cusp repair was performed in 55.4% of TAV. Freedom from reoperation was 90% and from AI >2+ was 88.4% at ten years, but preoperative severe AI requiring multiple cusp repair was associated with recurrent moderate-severe AI (44).
Although it is difficult to compare the results of different groups due to an unknown degree of selection bias, a clear theme is that cusp repair is durable when applied in the correct situations. A majority of those requiring repair do well with central plication and a highly select group benefit from other repair techniques. We recently reported our series, in which 22.7% of BAV had cusp repair. No patients required reoperation at five years and freedom from greater than mild AI was 92.1% vs. 100% (P=0.33) (11). Our group is conservative when it comes to cusp repair as we suspect that manipulation of the leaflet tissue may lead to inferior long-term durability, although longer follow-up is necessary to confirm.
VSRR with connective tissue disorder
VSRR has been studied extensively in patients with Marfan syndrome and other connective tissue disorders and has been found to be a safe alternative to valve-replacing aortic root surgery. As many of these patients are in the younger age spectrum, the ability to preserve the native aortic valve has clear potential benefit compared to replacement with a mechanical or bioprosthetic valve-conduit. Coselli et al. found in a multicenter study that VSRR had a higher cumulative incidence of adverse valve-related events, although this finding was driven mostly by valve dysfunction and AI. Despite some degree of valve deterioration, there was no difference in reintervention (45). However, a study from the Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions found that none of their valve-sparing patients required re-operative root surgery, but 11.5% of valve replacement patients did (46). Additionally, Price et al. found that VSRR was associated with fewer thromboembolic or hemorrhagic events, likely because these young patients often receive mechanical prostheses when valve replacement is necessary (47).
The durability of VSRR in Marfan patients is similar to VSRR in non-Marfan patients. Martens et al. showed an 86% and 80% freedom from aortic valve reoperation at ten and twenty years, respectively, which was similar to non-Marfan patients (48). Similarly, David et al. found that the rate of AI at fifteen years was 7.9% and only five of 146 patients required late reoperation (49). Many of the above findings apply to other connective tissue disorders as well, wherein the disorder affects the aortic tissue but not necessarily the aortic valve tissue. Seike et al. compared outcomes between Marfan syndrome and Loeys-Dietz syndrome patients and found comparable cumulative incidence of AI greater than 1+ and aortic valve reoperations. However, Loeys-Dietz patients had a stronger tendency toward late aortic dissection and higher incidence of aortic reoperation (50).
VSRR with acute type A aortic dissection
Valve-sparing root reimplantation has been used sparingly in patients with acute type A aortic dissection (ATAAD). However, the acuity with which most of these patients present limits the ability to perform a complex root replacement procedure. Tanaka et al. performed VSRR in 24 of 328 ATAAD with no in-hospital mortality but these were carefully selected patients with no organ malperfusion, compromised ventricular function or other serious systemic disease (51). The Emory group reported data on patients undergoing VSRR with “expanded indications” (ATAAD, 3+ or more AI or reoperation) with operative mortality of 2.2% and freedom from reoperation 96% at six years. The subgroup with ATAAD had 6.9% operative mortality highlighting the higher risk nature of this group (19). Sievers et al. reported excellent durability of VSRR with ATAAD with a cumulative incidence of reoperation of 13.4% at fifteen years but 30-day mortality was 15.9% (52). Similarly, the Leipzig group performed VSRR in 27 of 374 ATAAD with good mid-term survival of 80%, but hospital mortality was 15% (53). Our group takes a more conservative approach, similar to the Japanese group, in order to minimize operative mortality. Our priority is to survive the index operation. Of 343 ATAAD, root replacement was performed in 67 patients, nine of which were VSRR. Overall operative mortality was 5.6% with no deaths after VSRR (54). With ATAAD, we reserve complex repairs such as VSRR for select patients who are hemodynamically stable with no organ malperfusion, compromised cardiac function or other high risk comorbidities.
Long-term outcomes and durability
In the past few years, reports on the long-term durability of VSRR out to more than twenty years have become available. In the longest running series by David et al., 69.1% of patients were alive without aortic valve reoperation at twenty years. Cumulative risk of aortic valve reoperation was 6% and of moderate or severe AI was 10.2% (16). Several other groups have reported ten to fifteen year outcomes as well. The Brussels group had in-hospital mortality of 1%, ten year survival was 75%, and freedom from reoperation was 90% and freedom from greater than 2+ AI was 75% at ten years (44). Similarly, the group from Lille, France had survival of 82.9% at fifteen years, freedom from moderate or greater AI 74.3% at ten years, and freedom from valve reintervention 92.5% at fifteen years (18). The Hannover group showed a fifteen year survival of 65% and freedom from valve reoperation of 85% at fifteen years (55). Although some reimplanted valves develop late AI, few of them require valve reoperation.
Long-term results in studies comparing root replacement strategies offer additional insights. Ouzounian and colleagues used a propensity score to adjust for unbalanced variables in their analysis of 616 patients undergoing elective root surgery with either VSRR, bCVG or mCVG with follow-up to approximately ten years. All-cause mortality was comparable among the groups but both bCVG and mCVG were associated with increased major valve-related events (HR =3.4, P=0.005; and HR =5.2, P<0.001) compared to VSRR as well as increased mortality from cardiac cause (HR =7.0, P=0.001; and HR =6.4, P=0.003) (9). Compared to VSRR, bCVG procedures had an increased risk of reoperation on the valve (HR =6.9, P=0.003) and mCVG procedures had increased risk of anticoagulation related hemorrhage (HR =5.6, P=0.008). The risk of reoperation at year fifteen for reimplantation-VSRR in this study was similar to that of the mCVG group (1.1%) and significantly lower than that of the bCVG group (20%). Svensson and colleagues also report favorable durability in their evaluation comparing VSRR and CVG in 100 propensity-matched pairs, with only 2% of VSRR patients requiring reintervention at ten years compared to zero in the bCVG group (P=0.8) (15). Leontyev and colleagues used propensity-matching to evaluate long-term outcomes in 261 VSRR and 262 CVG patients. Survival and freedom from reoperation at ten years was 87.2% at 88.4%, respectively, with no difference between the two groups (56). Neither connective tissue disease nor bicuspid valve type were independent predictors of the need for reoperation. There was no difference in freedom from all valve-related events between the groups, but the CVG-treated patients did have a significantly higher rate of bleeding requiring hospitalization during follow-up (1.9% vs. 0%, P=0.025).
Conclusions
In summary, we pursue the VSRR approach in many patient cohorts but are more conservative than some groups in higher risk scenarios or when we suspect there is a higher likelihood of early structural degeneration. When evaluating a valve for preservation, significant calcifications, fenestrations or perforations will prompt conversion to the conduit valved graft technique. VSRR requiring cusp repair is usually performed only when a simple repair such as central plication is adequate as we suspect significant manipulation of the leaflet tissue may negatively affect long-term durability. These considerations apply to trileaflet and bicuspid valves, as well as patients with connective tissue disorders such as Marfan syndrome. We will perform VSRR in the setting of severe preoperative AI or severe root dilation if cusp quality and geometry is maintained, but favor conduit valved grafts for severe AI in the bicuspid valve or in trileaflet valves with compromise of the leaflets in any way. In higher risk scenarios such as ATAAD, we will only perform VSRR in patients who are hemodynamically stable and free from malperfusion, compromised cardiac function or other high-risk comorbidities. An ejection fraction of less than 40% will generally prompt a conduit valved graft strategy. Although we do not have a strict age cutoff for performing VSRR, we most often reserve this approach for patients under the age of seventy except in rare circumstances.
In conclusion, VSRR has matured into an excellent aortic root replacement option, especially in young patients with long life expectancy and favorable valve characteristics. The durability of reimplanted valves surpasses that of bioprosthetic valves, and they do not require lifelong anticoagulation. Although originally used mostly with normal aortic valves, the VSRR technique has been applied to many situations, including higher risk ones such as ATAAD and preoperative severe AI. Combining VSRR with proven cusp repair techniques has further increased the population of patients that VSRR can be applied to without significantly impacting durability. With long-term data now available, VSRR appears an ideal solution for many patients receiving care in specialized aortic centers. Older patients and those with higher risk comorbidities or unfavorable leaflet characteristics can also expect excellent outcomes and reasonable durability from the bCVG strategy. Mechanical CVG, once the gold standard for young patients needing aortic root replacement, remains a durable solution but with a price of late complications related to anticoagulation and thromboembolism.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Etz CD, Bischoff MS, Bodian C, et al. The Bentall procedure: is it the gold standard? A series of 597 consecutive cases. J Thorac Cardiovasc Surg 2010;140:S64-70; discussion S86-91. [Crossref] [PubMed]
- Crawford ES, Svensson LG, Coselli JS, et al. Surgical treatment of aneurysm and/or dissection of the ascending aorta, transverse aortic arch, and ascending aorta and transverse aortic arch. Factors influencing survival in 717 patients. J Thorac Cardiovasc Surg 1989;98:659-73; discussion 673-4. [Crossref] [PubMed]
- Kouchoukos NT, Wareing TH, Murphy SF, et al. Sixteen-year experience with aortic root replacement. Results of 172 operations. Ann Surg 1991;214:308-18; discussion 318-20. [Crossref] [PubMed]
- Cabrol C, Pavie A, Gandjbakhch I, et al. Complete replacement of the ascending aorta with reimplantation of the coronary arteries: new surgical approach. J Thorac Cardiovasc Surg 1981;81:309-15. [Crossref] [PubMed]
- De Paulis R, Scaffa R, Maselli D, et al. Valsalva graft in the Bentall procedure: from mechanical valve to the BioValsalva, world's first biological aortic conduit. Surg Technol Int 2008;17:216-21. [PubMed]
- Kunihara T, Ichihara N, Miyata H, et al. Valve-sparing root replacement and composite valve graft replacement in patients with aortic regurgitation: From the Japan Cardiovascular Surgery Database. J Thorac Cardiovasc Surg 2019;158:1501-1511.e6. [Crossref] [PubMed]
- Gaudino M, Lau C, Munjal M, et al. Contemporary outcomes of surgery for aortic root aneurysms: A propensity-matched comparison of valve-sparing and composite valve graft replacement. J Thorac Cardiovasc Surg 2015;150:1120-9.e1. [Crossref] [PubMed]
- Ouzounian M, Rao V, Manlhiot C, et al. Valve-Sparing Root Replacement Compared With Composite Valve Graft Procedures in Patients With Aortic Root Dilation. J Am Coll Cardiol 2016;68:1838-47. [Crossref] [PubMed]
- Chiang YP, Chikwe J, Moskowitz AJ, et al. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA 2014;312:1323-9. [Crossref] [PubMed]
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622. [Crossref] [PubMed]
- Lau C, Wingo M, Rahouma M, et al. Valve-sparing root replacement in patients with bicuspid aortopathy: An analysis of cusp repair strategy and valve durability. J Thorac Cardiovasc Surg 2021;161:469-78. [Crossref] [PubMed]
- Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol 2012;60:1156-62. [Crossref] [PubMed]
- Stamou SC, Williams ML, Gunn TM, et al. Aortic root surgery in the United States: a report from the Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2015;149:116-22.e4. [Crossref] [PubMed]
- Svensson LG, Rosinski BF, Tucker NJ, et al. Comparison of Outcomes of Patients Undergoing Reimplantation versus Bentall Root Procedure. Aorta (Stamford) 2022;10:57-68. [Crossref] [PubMed]
- David TE, David CM, Ouzounian M, et al. A progress report on reimplantation of the aortic valve. J Thorac Cardiovasc Surg 2021;161:890-899.e1. [Crossref] [PubMed]
- Shrestha M, Boethig D, Krüger H, et al. Valve-sparing aortic root replacement using a straight tube graft (David I procedure). J Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Manganiello S, Soquet J, Mugnier A, et al. David Procedure: A 21-year Experience With 300 Patients. Ann Thorac Surg 2023;115:1403-10. [Crossref] [PubMed]
- Leshnower BG, Guyton RA, Myung RJ, et al. Expanding the indications for the David V aortic root replacement: early results. J Thorac Cardiovasc Surg 2012;143:879-84. [Crossref] [PubMed]
- Di Franco A, Rong LQ, Munjal M, et al. Aortic symmetry index: Initial validation of a novel preoperative predictor of recurrent aortic insufficiency after valve-sparing aortic root reconstruction. J Thorac Cardiovasc Surg 2018;156:1393-4. [Crossref] [PubMed]
- De Paulis R, Scaffa R, Nardella S, et al. Use of the Valsalva graft and long-term follow-up. J Thorac Cardiovasc Surg 2010;140:S23-7; discussion S45-51. [Crossref] [PubMed]
- Kallenbach K, Karck M, Leyh RG, et al. Valve-sparing aortic root reconstruction in patients with significant aortic insufficiency. Ann Thorac Surg 2002;74:S1765-8; discussion S1792-9. [Crossref] [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. Effects of preoperative aortic insufficiency on outcome after aortic valve-sparing surgery. Circulation 2009;120:S120-6. [Crossref] [PubMed]
- Mastrobuoni S, de Kerchove L, Navarra E, et al. Long-term experience with valve-sparing reimplantation technique for the treatment of aortic aneurysm and aortic regurgitation. J Thorac Cardiovasc Surg 2019;158:14-23. [Crossref] [PubMed]
- McCarthy FH, Bavaria JE, McDermott KM, et al. At the Root of the Repair Debate: Outcomes After Elective Aortic Root Replacements for Aortic Insufficiency With Aneurysm. Ann Thorac Surg 2016;102:1199-205. [Crossref] [PubMed]
- Deas DS Jr, Lou X, Leshnower BG, et al. Fifteen Years of Aortic Valve-sparing Root Replacement and Impact of Eccentric Jets on Late Outcomes. Ann Thorac Surg 2021;112:1901-7. [Crossref] [PubMed]
- Leyh RG, Kallenbach K, Karck M, et al. Impact of preoperative aortic root diameter on long-term aortic valve function after valve sparing aortic root reimplantation. Circulation 2003;108:II285-90. [Crossref] [PubMed]
- Kari FA, Siepe M, Rylski B, et al. Aortic valve reimplantation for large root aneurysm and high-grade aortic regurgitation: incidence and implications of additional cusp and commissure repair. Interact Cardiovasc Thorac Surg 2015;20:611-5. [Crossref] [PubMed]
- Ouzounian M, Feindel CM, Manlhiot C, et al. Valve-sparing root replacement in patients with bicuspid versus tricuspid aortic valves. J Thorac Cardiovasc Surg 2019;158:1-9. [Crossref] [PubMed]
- Alsoufi B, Borger MA, Armstrong S, et al. Results of valve preservation and repair for bicuspid aortic valve insufficiency. J Heart Valve Dis 2005;14:752-8; discussion 758-9. [PubMed]
- Sareyyupoglu B, Suri RM, Schaff HV, et al. Survival and reoperation risk following bicuspid aortic valve-sparing root replacement. J Heart Valve Dis 2009;18:1-8. [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011;142:1430-8. [Crossref] [PubMed]
- Habertheuer A, Milewski RK, Bavaria JE, et al. Predictors of Recurrent Aortic Insufficiency in Type I Bicuspid Aortic Valve Repair. Ann Thorac Surg 2018;106:1316-24. [Crossref] [PubMed]
- Kari FA, Liang DH, Kvitting JP, et al. Tirone David valve-sparing aortic root replacement and cusp repair for bicuspid aortic valve disease. J Thorac Cardiovasc Surg 2013;145:S35-40.e1-2.
- Bavaria JE, Desai N, Szeto WY, et al. Valve-sparing root reimplantation and leaflet repair in a bicuspid aortic valve: comparison with the 3-cusp David procedure. J Thorac Cardiovasc Surg 2015;149:S22-8. [Crossref] [PubMed]
- Liebrich M, Kruszynski MK, Roser D, et al. The David procedure in different valve pathologies: a single-center experience in 236 patients. Ann Thorac Surg 2013;95:71-6. [Crossref] [PubMed]
- Kayatta MO, Leshnower BG, McPherson L, et al. Valve Sparing Root Replacement Provides Similar Midterm Outcomes in Bicuspid and Trileaflet Valves. Ann Thorac Surg 2019;107:54-60. [Crossref] [PubMed]
- Mokashi SA, Rosinski BF, Desai MY, et al. Aortic root replacement with bicuspid valve reimplantation: Are outcomes and valve durability comparable to those of tricuspid valve reimplantation? J Thorac Cardiovasc Surg 2022;163:51-63.e5. [Crossref] [PubMed]
- Klotz S, Stock S, Sievers HH, et al. Survival and reoperation pattern after 20 years of experience with aortic valve-sparing root replacement in patients with tricuspid and bicuspid valves. J Thorac Cardiovasc Surg 2018;155:1403-1411.e1. [Crossref] [PubMed]
- Beckmann E, Martens A, Krüger H, et al. Aortic valve-sparing root replacement in patients with bicuspid aortic valve: long-term outcome with the David I procedure over 20 years. Eur J Cardiothorac Surg 2020;58:86-93. [Crossref] [PubMed]
- David TE, David CM, Feindel CM, et al. Reimplantation of the aortic valve at 20 years. J Thorac Cardiovasc Surg 2017;153:232-8. [Crossref] [PubMed]
- Settepani F, Cappai A, Basciu A, et al. Impact of Cusp Repair on Reoperation Risk After the David Procedure. Ann Thorac Surg 2016;102:1503-11. [Crossref] [PubMed]
- Liebrich M, Charitos E, Stadler C, et al. Additional cusp reconstruction does not compromise valve durability and mid-term survival after the David procedure: results from 449 patients. Eur J Cardiothorac Surg 2020;58:1072-9. [Crossref] [PubMed]
- Tamer S, Mastrobuoni S, Lemaire G, et al. Two decades of valve-sparing root reimplantation in tricuspid aortic valve: impact of aortic regurgitation and cusp repair. Eur J Cardiothorac Surg 2021;59:1069-76. [Crossref] [PubMed]
- Coselli JS, Volguina IV, LeMaire SA, et al. Midterm outcomes of aortic root surgery in patients with Marfan syndrome: A prospective, multicenter, comparative study. J Thorac Cardiovasc Surg. 2023;165:1790-9.e12. [Crossref] [PubMed]
- Song HK, Preiss LR, Maslen CL, et al. Valve-sparing aortic root replacement in patients with Marfan syndrome enrolled in the National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions. J Heart Valve Dis 2014;23:292-8. [PubMed]
- Price J, Magruder JT, Young A, et al. Long-term outcomes of aortic root operations for Marfan syndrome: A comparison of Bentall versus aortic valve-sparing procedures. J Thorac Cardiovasc Surg 2016;151:330-6. [Crossref] [PubMed]
- Martens A, Beckmann E, Kaufeld T, et al. Valve-sparing aortic root replacement (David I procedure) in Marfan disease: single-centre 20-year experience in more than 100 patients†. Eur J Cardiothorac Surg 2019;55:476-83. [Crossref] [PubMed]
- David TE, David CM, Manlhiot C, et al. Outcomes of Aortic Valve-Sparing Operations in Marfan Syndrome. J Am Coll Cardiol 2015;66:1445-53. [Crossref] [PubMed]
- Seike Y, Yokawa K, Koizumi S, et al. Long-term durability of a reimplantation valve-sparing aortic root replacement can be expected in both Marfan syndrome and Loeys-Dietz syndrome. Eur J Cardiothorac Surg 2022;61:1318-25. [Crossref] [PubMed]
- Tanaka H, Ikeno Y, Abe N, et al. Outcomes of valve-sparing root replacement in acute Type A aortic dissection. Eur J Cardiothorac Surg 2018;53:1021-6. [Crossref] [PubMed]
- Sievers HH, Richardt D, Diwoky M, et al. Survival and reoperation after valve-sparing root replacement and root repair in acute type A dissection. J Thorac Cardiovasc Surg 2018;156:2076-2082.e2. [Crossref] [PubMed]
- Subramanian S, Leontyev S, Borger MA, et al. Valve-sparing root reconstruction does not compromise survival in acute type A aortic dissection. Ann Thorac Surg 2012;94:1230-4. [Crossref] [PubMed]
- Lau C, Robinson NB, Farrington WJ, et al. A tailored strategy for repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2022;164:1698-1707.e3. [Crossref] [PubMed]
- Beckmann E, Martens A, Krüger H, et al. Aortic valve-sparing root replacement with Tirone E. David's reimplantation technique: single-centre 25-year experience. Eur J Cardiothorac Surg 2021;60:642-8. [Crossref] [PubMed]
- Leontyev S, Schamberger L, Davierwala PM, et al. Early and Late Results After David vs Bentall Procedure: A Propensity Matched Analysis. Ann Thorac Surg 2020;110:120-6. [Crossref] [PubMed]







