Three decades of reimplantation of the aortic valve—the Brussels experience
Introduction
The introduction of valve-sparing root replacement techniques has revolutionized the treatment of aortic root and aortic valve disease (1,2). Previously, the aortic root was commonly replaced together with the aortic valve, by means of a composite graft (aortic root + aortic valve) replacement (3). This was the preferred surgical approach, regardless of the presence of a normal or dysfunctional valve.
However, over the last three decades, the importance of native valve-preservation has become increasingly evident. Not only does it obviate the need for anticoagulation and patient compliance with improved quality of life, but it also decreases co-morbidities such as bleeding, strokes and endocarditis. Most importantly, we now know that valve-preservation restores normal life expectancy (4,5). Both the remodeling (Yacoub) and reimplantation (David) techniques, have been refined, with different iterations of the reimplantation technique and additions to the remodeling technique (annuloplasty) over time (6,7).
Although, early on, valvular disease such as aortic regurgitation was considered an absolute and later a relative contraindication for the reimplantation technique, the El Khoury classification (8) for aortic regurgitation has helped us understand the mechanism of aortic regurgitation, and has thus afforded us the ability to now perform valve-preservation despite a severely dysfunctional aortic valve. Aortic valve disease can be due to abnormalities of the valve itself or due to abnormalities of the aortic root, the functional aortic annulus [virtual basal ring (VBR) to Sino-tubular junction (STJ)]. Moreover, the aortic valve can present with different phenotypes, and we have learned that valve preservation is not only feasible with tricuspid aortic valves (TAV), but also the entire gamut of valve phenotypes (5,9-15). Our group has developed a new iteration of the David technique to specifically accommodate the unique features of the bicuspid aortic valve (BAV), and to create a more symmetric valve (The El Khoury 180° reimplantation technique) (11). This has demonstrated excellent long-term results, with improved freedom from reoperation over time (5). As previously mentioned, aortic regurgitation can be related to valvular abnormalities and/or root abnormalities (8). Root abnormalities are mainly secondary to root enlargement and this can occur at any level; at the VBR, STJ or in-between. Hence, one of the major advantages of the reimplantation technique is not only restoration of normal root dimensions, but also stabilization of the entire functional aortic annulus (FAA).
In 2008, we started performing the reimplantation technique even in patients without a significant root enlargement (<4.5 cm). In reality, many of these patients, with so called isolated chronic AR, frequently present with annuloaortic ectasia and some degree of root enlargement (≥40 mm). The possibility of remodeling and stabilizing the FAA at all levels, and reshaping valve geometry (in BAV), are the main reasons that have led us to use the reimplantation technique in these non-classical indications.
Herein, we sought to analyze and summarize our experience with the reimplantation technique over the last three decades for the following three distinct indications: root aneurysm without AR (grade ≤1+), root aneurysm with AR (grade >1+) and isolated chronic AR (root <45 mm).
Methods
Study design
All adult patients (≥18 years), who underwent the valve-sparing root replacement with reimplantation technique (VSRR) at our institution (Cliniques Universitaires Saint-Luc, Brussels, Belgium) between March 1998 and January 2022 were included in this analysis. The indication for VSRR operation was aortic root aneurysm and/or severe AR. Clinical follow-up data was collected by telephone or by the referring cardiologist. Follow-up clinical and echocardiographic data was collected from hospital records and cardiologist reports. Serial standardized echocardiogram examinations were routinely performed at our institution. Clinical data was reported according to the 2008 Society of Thoracic Surgeons/American Association for Thoracic Surgery/European Association for Cardio-Thoracic Surgery guidelines (16). Early mortality was defined as any death occurring during hospital stay or during the first 60 days after the operation; any other mortality events were considered late deaths. Patient data was extracted from our institution’s prospective database for aortic valve repair, and this study was approved by the ethics review board of our academic institution, Université Catholique de Louvain (ID Brussels: 2013/03JUI/356).
Surgical technique
Our technique has previously been described in detail for tricuspid and bicuspid aortic valves (11,17). In brief, after the aorta is cross-clamped and cardioplegic arrest of the heart is achieved, a horizontal aortotomy, 1 cm above the STJ, is performed and the valve is carefully examined. External root dissection and preparation is followed by excision of the Sinuses of Valsalva. The proximal suture line is performed with 10 to 12 pledgetted horizontal mattress sutures at the level of the VBR. The size of the vascular graft is then chosen by measuring the height of the commissure at the level of the non-/left-coronary commissure (14,18). After completion of the proximal suture line, the valve is reimplanted within the graft starting from the three commissures.
The valve is then reexamined and any residual prolapse or other lesions are addressed and corrected. The techniques of cusp repair have been previously described and consist mainly of free margin plication and free margin resuspension (19). The special considerations in case of BAV have been reported elsewhere (5,11,14). In summary, in type A BAV (symmetric, commissural orientation: 160°–180°), the symmetry of cusps and sinuses is respected or enhanced with the 180° reimplantation technique. In type B BAV (asymmetric, commissural orientation: 140°–159°) with a restrictive raphe and a lack of cusp tissue of the conjoined cusp, the valve is made symmetric by means of a selective annuloplasty. The commissures are generally, with a few exceptions, reimplanted at 180°. The root attachment of the raphe is resected and leaflet fibrous thickening is thinned. The non-fused portion of the conjoined cusp is closed with direct suturing thereof, so as to increase the geometric height of the leaflet and at the same time treat its prolapse.
Statistical analysis
Categorical data is presented as counts with proportions. Continuous data is presented as means (standard deviation; range) when normally distributed, or medians (interquartile range) when not normally distributed. For categorical data, the χ2 test or the Fisher exact test was used for comparison between groups. For continuous data, an unpaired t-test or Mann-Whitney U test, depending on distribution, was used. Univariable logistic regression analysis was performed to study potential variables affecting early mortality. Candidate variables with a P value of <0.10, or those that were clinically relevant, were tested in a multivariable model. Survival and freedom from valve reintervention were analyzed with the Kaplan-Meier estimator, and for analysis of association of variables (i.e., predictors) with outcomes (P value of <0.1 or clinically relevant), the Cox regression model was used. The proportional hazard assumption was met by visual inspection of log-log curves. All tests were performed 2-sided, and a P value of <0.05 was considered statistically significant. For the hazard of late death, the following variables were considered: age, gender, valve phenotype, BMI, preoperative New York Heart Association (NYHA), preoperative left ventricle ejection fraction, left ventricular end-diastolic dimension (LVEDD), chronic renal failure, pulmonary hypertension, chronic obstructive pulmonary disease, peripheral artery disease, diabetes, indication for surgery (i.e., Aneurysm, Aneurysm + AR or isolated AR), previous cardiac surgery, connective tissue disorder, type A acute aortic dissection, concomitant procedures, and cardiac reoperation. To account for informative censoring, survival and freedom from reintervention were presented with the cumulative incidence function, from a competing risk analysis (using the R “cmprsk” package) and predictors of late AV reintervention were analyzed accounting for the competing risk of death with competing-risk regression model with the Fine-Gray method (20). For statistical analysis, R (version 4.2.0, available at: www.r-project.org) and GraphPad Prism version 9.3.1 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com, were used.
Results
Demographics
During the study period, a total of 652 patients underwent a valve-sparing root replacement operation with the reimplantation technique, at a single institution. Reimplantation was performed for aortic aneurysm without AR in 213 patients, for aortic aneurysm with AR in 289 patients, and for isolated AR in 150 patients. The median follow-up time was 6.1 years (IQR 2.1–10.0 years) [aneurysm: 5.9 years (IQR 1.7–10.2 years); aneurysm + AR: 6.6 years (IQR 2.8–10.5 years); isolated AR: 5.3 years (IQR 1.6–8.8 years)]. Patient characteristics and perioperative data is summarized in Table 1.
Table 1
| Characteristic | Aneurysm (n=213) | Aneurysm + AR (n=289) | Isolated AR (n=150) | P value |
|---|---|---|---|---|
| Age years, mean [SD] | 48.8 (13.6) | 53.1 (14.0) | 43.7 (14.2) | 0.04 |
| Male, n (%) | 188 (88.3) | 260 (90.0) | 140 (93.3) | 0.28 |
| NYHA class, n (%) | <0.001 | |||
| I | 174 (81.7) | 163 (56.4) | 78 (52.0) | |
| II | 34 (16.0) | 93 (32.2) | 59 (39.3) | |
| III | 4 (1.9) | 32 (11.1) | 12 (8.0) | |
| IV | 1 (0.5) | 1 (0.3) | 1 (0.7) | |
| Previous cardiac surgery, n (%) | 22 (10.3) | 37 (12.8) | 18 (12.0) | 0.18 |
| Hypertension, n (%) | 42 (19.7) | 60 (20.8) | 37 (24.7) | 0.50 |
| Coronary artery disease, n (%) | 16 (7.5) | 20 (6.9) | 8 (5.3) | 0.71 |
| CTD, n (%) | 36 (16.9) | 18 (6.2) | 1 (0.7) | <0.001 |
| LVEF, n (%) | <0.001 | |||
| ≥50% | 202 (94.8) | 241 (83.4) | 127 (84.7) | |
| 31–49% | 10 (4.7) | 41 (14.2) | 23 (15.3) | |
| ≤30% | 1 (0.5) | 7 (2.4) | 0 | |
| LVEDD mm, mean [SD] | 53 [5] | 61 [8] | 63 [7] | 0.02 |
| Valve phenotype, n (%) | <0.001 | |||
| TAV | 144 (67.6) | 185 (64.0) | 53 (35.3) | |
| BAV | 69 (32.4) | 102 (35.3) | 94 (62.7) | |
| Unicuspid/quadricuspid | 0 | 2 (0.7) | 3 (2.0) | |
| Aortic regurgitation, n (%) | <0.001 | |||
| 0–1 | 100 | 3 (1.0) | 4 (2.7) | |
| 2 | 0 | 94 (32.6) | 7 (4.8) | |
| 3 | 0 | 119 (41.3) | 67 (45.6) | |
| 4 | 0 | 72 (25.0) | 69 (46.9) | |
| CPB time, minutes, mean [SD] | 146 [36] | 148 [35] | 156 [35] | 0.42 |
| Cross clamp time, mean [SD] | 123 [27] | 123 [30] | 129 [25] | 0.28 |
AR, aortic regurgitation; SD, standard deviation; NYHA, New York Heart Association; CTD, connective tissue disorder; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; TAV, tricuspid aortic valves; BAV, bicuspid aortic valve; CPB, cardiopulmonary bypass.
Outcomes
Early outcomes
Three patients died in the early postoperative period (0.5%). Two of these patients underwent reimplantation for a type A aortic dissection.
One patient required early (during the same hospitalization) reoperation of the aortic valve, due to progressive aortic valve regurgitation on follow-up echocardiography. This patient underwent a successful re-repair.
Late survival
A total of 65 patients died during follow-up, 23 due to cardiac (5 valve-related) and 42 due to non-cardiac deaths. Cumulative survival was 95.4% (95% CI: 92.9–97.0%) after 5 years, 84.8% (80.0–88.5%) after 10 years, and 79.5% (73.3–84.5%) after 12 years, which was comparable to the age-matched Belgian population (Figure 1A shows overall survival compared to the age matched Belgian population).
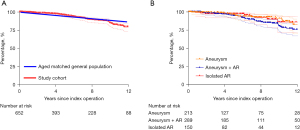
Survival was not significantly different between the three groups (P=0.2) as displayed in Figure 1B. A univariate analysis identified the following variables as predictors for late mortality: age hazard ratio (HR) 1.07 (P<0.001), female gender HR 0.40 (P=0.03), BAV HR 0.20 (P=0.01), peripheral artery disease HR 4.98 (P=0.04), ATAAD HR 3.89 (P=0.004), and endocarditis HR 3.49 (P=0.08). After adjustment in a multivariable Cox regression model, only older age (HR 1.06, P≤0.001) and male gender (HR 2.1, P=0.02) were associated with late mortality.
Late reoperations on the aortic valve
A total of 44 patients underwent reoperation on the aortic valve. Reason for reoperations were recurrent severe AR (N=20), severe aortic stenosis (N=14), aortic valve endocarditis (N=7), and severe mixed AR and stenosis (N=3). Failure due to recurrent severe AR occurred in 15 TAV and 4 BAV (and 1 unicuspid); failure due to aortic stenosis and mixed AR and stenosis occurred in 2 TAV and 15 BAV. Endocarditis occurred in 5 TAV and 2 BAV. Freedom from reoperation on the aortic valve at 5 years was 96.2% (95% CI: 93.8–97.7%), and 90.4% (95% CI: 87.4–94.2%) at 12 years (Figure 2).
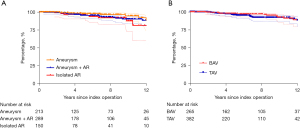
Freedom from reoperation was not different between the three groups (P=0.14) (Figure 2A), nor between tricuspid valves and bicuspid aortic valves (P=0.3) (Figure 2B).
A univariable analysis identified the following variables as predictors for late reoperation: age sub-distribution hazard ratio (SHR) 0.96; 95% CI: 0.93–0.99 (P=0.006); preoperative LVEDD SHR 1.04; 95% CI: 0.98–1.12 (P=0.04), and connective tissue disorder SHR 2.55; 95% CI: 1.1–8.16 (P=0.05).
After adjustment in a multivariable Cox regression, only age HR 0.96; 95% CI: 0.93–0.98 (P=0.001) and preoperative LVEDD HR 1.05; 95% CI: 1.01–1.10 (P=0.03) were associated with late reoperation.
Figure 3 shows a competing risk analysis of overall reoperation-free survival versus the competing risk of reoperation or death.
Other valve related outcomes
Figure 4 shows freedom from aortic valve regurgitation of grade 3 or more during follow-up, examined by echocardiography. Furthermore, Table 2 displays the linearized occurrence rates for survival, reoperation on aortic valve and valve-related events of bleeding, thromboembolism, and endocarditis.
Table 2
| Outcome | Aneurysm | Aneurysm + AR | Isolated AR |
|---|---|---|---|
| Total follow-up, patients/year | 1,342 | 2,052 | 855 |
| Late death, %/year | 1.56 | 2.14 | 1.17 |
| Reintervention, %/year | 0.60 | 1.17 | 1.40 |
| Thromboembolism, %/year | 0.15 | 0.23 | 0.10 |
| Bleeding, %/year | 0.05 | 0.21 | 0.08 |
| Endocarditis, %/year | 0.30 | 0.15 | 0.00 |
LOR = events per patient/year of follow-up. AR, aortic regurgitation; LOR, linearized occurrence rates.
Discussion
The reimplantation technique has evolved, over time, to become the cornerstone of our aortic valve-preservation program (valve-sparing root replacement and/or aortic valve repair). This evolution is based on our learning curve over the last three decades, when different techniques were utilized during different time periods. The reimplantation technique provided the best long-term results, was standardized for the most common aortic valve phenotypes (TAV or BAV) and provided the best stabilization of the functional aortic annulus at every level [VBR, STJ, and in-between (including the interleaflet triangles and Sinuses of Valsalva)], with functional exclusion of all areas of the aortic annulus at risk for re-dilation during long-term follow-up (5,10).
The results presented here constitute one of the largest cohorts of patients who have been treated with the reimplantation technique for various aortic valve and/or aortic root pathologies to date.
We have previously reported that valve-preservation restores normal life-expectancy (5), which is also confirmed in this study, where survival at 12-year was comparable to an age-matched Belgian population. The cumulative survival of our entire patient cohort, was 95.4%, 84.8% and 79.5% for 5-, 10- and 12-year survival, respectively. There was no difference in survival between the three study groups, who all underwent root replacement with the reimplantation technique. The most common reason for late reoperation was recurrent severe AR, in 3% of the entire study cohort (most occurred in TAV) followed by severe aortic stenosis at 2% (most occurred in BAV). Risk factors for aortic stenosis have been previously described in detail by our group (21).
Moreover, freedom from reoperation was no different between the three groups. The Rome group has reported comparable results in a cohort of 124 patients with 5-, 10-, and 13-year survival rates of 94.4%, 90.5% and 81.4%, respectively (22). Freedom from moderate to severe aortic regurgitation was 94.1% and 87.1% at 5 and 10 years, respectively. As in the Rome group, we also predominantly use the Gelweave™ Valsalva graft (Terumo Aortic, Renfrewshire, UK), and only in tricuspid aortic valves with irregular commissural heights, will we sometimes use a straight tube graft instead.
We have found that only older age and male gender were risk factors for late mortality in our multivariable analysis.
In 2020, the Homburg group reported 5-, 10- and 15-year survival rates of 94.3%, 89.6% and 86.1% for their remodeling technique, respectively (23). This was in 1,038 patients. Freedom from reoperation was 94% at 10- and 15-year for TAV, and 88% and 80% for BAV, respectively. Overall freedom from reoperation in our entire study cohort was 96.2% and 90.4% at 5- and 12-year, respectively. There was no difference between TAV and BAV. Risk factors for late reoperation in our multivariable analysis were only age and preoperative LVEDD.
In the past, the question arose of why we sometimes perform reimplantation in patients with aortic regurgitation and a normal aortic root (24). The reason is that in our experience, the reimplantation technique has provided the best long-term results in patients who undergo aortic valve repair. This, we believe, is secondary to the comprehensive annuloplasty of the entire functional aortic annulus, which in turn provides superior stabilization of the aortic valve with excellent long-term durability, as also shown in this study. However, there is one caveat to this: we do not take root replacement in normal roots lightly. In these patients, we have to be absolutely sure that aortic valve repair will be successful in each individual patient, and hence, this then justifies our aggressive approach where the benefits of valve preservation outweigh the risks of resecting a normal root. Getting to this point, however, requires a high level of surgical experience and expertise, and should be approached with humility.
Our technique for sizing the prosthetic graft is very standardized, has been reported by our group on multiple occasions, and is well known in the surgical community (18). In brief, we size the graft by measuring the height of the posterior commissure (non/left). Other groups, such as the Toronto or Rome group, have also reported different strategies. In general, we recommend choosing the larger graft size when deciding between two graft-sizes. Effectively, leaflet free margins are elongated in chronically dilated aortic roots. Therefore, using a larger graft will somewhat mitigate a possible prolapse with smaller graft sizes. Oversizing the graft, however, may potentially render the valve leaflets more restrictive. Therefore, careful graft sizing and leaflet assessment is important. A possible prolapse can be remedied by performing a central leaflet plication. We do use calipers to assess effective height after the repair and measure the geometric height with a straight metal ruler to assess the valve and quantity of leaflet tissues before the repair. Moreover, with our modified reimplantation technique for BAV (the 180° reimplantation technique), we improve mobility of the conjoined leaflet, and relatively increase the valve orifice area that is covered by the non-fused and thus more mobile cusp (11,14). This is achieved through a selective annuloplasty and reimplantation of the two commissures at 180° (modification of valve geometry), with a previously reported 12-year survival of 94% and freedom from AR >2, of 97% (5).
A recent multi-center study (Aviator Registry) demonstrated that valve sparing procedures in patients with aortic root aneurysm with or without aortic regurgitation had excellent mid-term results compared to composite valve replacements (25). This was also described by the Toronto group, which showed that aortic valve-sparing procedures were associated with reduced cardiac mortality and valve-related complications compared to biologic or mechanical composite graft replacement (26). In Marfan syndrome, reimplantation of the aortic valve was associated with a lower risk of aortic valve reoperations and aortic insufficiency than the remodeling technique during a 20-year follow-up (27).
Conclusions
Our approach to valve-sparing surgery is centered around the reimplantation technique. This technique provides support of the entire functional aortic annulus and is safe and reproducible. Our long-term data supports our approach, with long-term survival that mirrors that of the general population. In other words: it restores life-expectancy.
At our center, we consider the reimplantation technique the superior technique for valve-sparing procedures (10), although good results have also been reported with the remodeling technique, when an extra annuloplasty is added at the level of the surgical annulus (VBR) (23,28). Some groups have even added an additional annuloplasty at the level of the STJ (29). Nonetheless, with the reimplantation procedure, the stabilization of the VBR and STJ is already an integral part of the procedure (11). We also agree with the Toronto group, that especially in patients with connective tissue disorders, reimplantation should be the preferred technique; since it functionally and effectively excludes all tissues at risk for future dilation (27,30).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg 1993;105:435-8. [Crossref] [PubMed]
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622. [Crossref] [PubMed]
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Tamer S, Mastrobuoni S, Lemaire G, et al. Two decades of valve-sparing root reimplantation in tricuspid aortic valve: impact of aortic regurgitation and cusp repair. Eur J Cardiothorac Surg 2021;59:1069-76. [Crossref] [PubMed]
- de Meester C, Vanovershelde JL, Jahanyar J, et al. Long-term durability of bicuspid aortic valve repair: a comparison of 2 annuloplasty techniques. Eur J Cardiothorac Surg 2021;60:286-94. [Crossref] [PubMed]
- Miller DC. Valve-sparing aortic root replacement in patients with the Marfan syndrome. J Thorac Cardiovasc Surg 2003;125:773-8. [Crossref] [PubMed]
- Aicher D, Schneider U, Schmied W, et al. Early results with annular support in reconstruction of the bicuspid aortic valve. J Thorac Cardiovasc Surg 2013;145:S30-4. [Crossref] [PubMed]
- El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005;20:115-21. [Crossref] [PubMed]
- Klepper M, Jahanyar J, Aphram G, et al. A rare case of pseudo-quadricuspid aortic valve repair. JTCVS Tech 2022;11:1-4. [Crossref] [PubMed]
- Jahanyar J, El Khoury G, de Kerchove L. Reimplantation should be the gold standard to treat the regurgitant bicuspid aortic valve. JTCVS Tech 2022;13:42-3. [Crossref] [PubMed]
- Jahanyar J, de Kerchove L, El Khoury G. Bicuspid aortic valve repair: the 180°-Reimplantation technique. Ann Cardiothorac Surg 2022;11:473-81. [Crossref] [PubMed]
- Jahanyar J, Aphram G, Munoz DE, et al. Congenital unicuspid aortic valve repair without cusp patch augmentation. J Card Surg 2022;37:2477-80. [Crossref] [PubMed]
- Lorenz V, Gonthier S, Jahanyar J, et al. Original repair after Ross failure: A case report of bicuspidized unicuspid autograft. JTCVS Tech 2021;8:141-3. [Crossref] [PubMed]
- Jahanyar J, El Khoury G, de Kerchove L. Commissural geometry and cusp fusion insights to guide bicuspid aortic valve repair. JTCVS Tech 2021;7:83-92. [Crossref] [PubMed]
- Mastrobuoni S, Aphram G, Tamer S, et al. Quadricuspid aortic valve repair. Ann Cardiothorac Surg 2019;8:433-5. [Crossref] [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;85:1490-5. [Crossref] [PubMed]
- de Kerchove L, Mosala Nezhad Z, Boodhwani M, et al. How to perform valve sparing reimplantation in a tricuspid aortic valve. Ann Cardiothorac Surg 2013;2:105-12. [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. A new simple and objective method for graft sizing in valve-sparing root replacement using the reimplantation technique. Ann Thorac Surg 2011;92:749-51. [Crossref] [PubMed]
- de Kerchove L, Glineur D, Poncelet A, et al. Repair of aortic leaflet prolapse: a ten-year experience. Eur J Cardiothorac Surg 2008;34:785-91. [Crossref] [PubMed]
- Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc 1999;94:496-509. [Crossref]
- Spadaccio C, Nenna A, Henkens A, et al. Predictors of long-term stenosis in bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2022;S0022-5223(22)00502-5.
- De Paulis R, Chirichilli I, Scaffa R, et al. Long-term results of the valve reimplantation technique using a graft with sinuses. J Thorac Cardiovasc Surg 2016;151:112-9. [Crossref] [PubMed]
- Miyahara S, Karliova I, Giebels C, et al. Aortic root remodeling in bicuspid and tricuspid aortic valves-long-term results. Indian J Thorac Cardiovasc Surg 2020;36:81-7. [Crossref] [PubMed]
- Mastrobuoni S, de Kerchove L, Navarra E, et al. Long-term experience with valve-sparing reimplantation technique for the treatment of aortic aneurysm and aortic regurgitation. J Thorac Cardiovasc Surg 2019;158:14-23. [Crossref] [PubMed]
- Arabkhani B, Klautz RJM, de Heer F, et al. A multicenter, propensity-score matched analysis comparing valve-sparing approach to valve replacement in aortic root aneurysm: Insight from AVIATOR database. Eur J Cardiothorac Surg 2023;63:ezac514. [Crossref] [PubMed]
- Ouzounian M, Rao V, Manlhiot C, et al. Valve-Sparing Root Replacement Compared With Composite Valve Graft Procedures in Patients With Aortic Root Dilation. J Am Coll Cardiol 2016;68:1838-47. [Crossref] [PubMed]
- Elbatarny M, David TE, David CM, et al. Improved Outcomes of Reimplantation vs Remodeling in Marfan Syndrome: A Propensity-Matched Study. Ann Thorac Surg 2023;115:576-82. [Crossref] [PubMed]
- Schneider U, Aicher D, Miura Y, et al. Suture Annuloplasty in Aortic Valve Repair. Ann Thorac Surg 2016;101:783-5. [Crossref] [PubMed]
- Lansac E, Di Centa I, Sleilaty G, et al. Remodeling root repair with an external aortic ring annuloplasty. J Thorac Cardiovasc Surg 2017;153:1033-42. [Crossref] [PubMed]
- Jahanyar J, de Kerchove L, Munoz DE, et al. Twenty-year follow-up after valve-sparing aortic root replacement with the Yacoub or David procedure in Marfan patients. JTCVS Open 2021;7:47-9. [Crossref] [PubMed]








