Valve-sparing root replacement—reimplantation technique
Introduction
Notable advancements in aortic root surgery over the past thirty years have centered on native aortic valve preservation. With the first Bentall Procedure described in 1968 by Hugh Bentall and Antony DeBono (1), the “bio Bentall” became a popular option in the 1990s to avoid anticoagulation. Over the past three decades, valve sparing root replacement (VSRR) has provided an option for treating aortic root pathology which avoids anticoagulation as well as potential early structural deterioration of a bioprosthetic valve. The two most common clinical settings where patients’ aortic root pathology is considered for VSRR are connective tissue diseases (Marfan syndrome, Loeys-Dietz syndrome) and bicuspid aortic valve (BAV) syndromes (2).
There are two primary techniques for performing VSRR. In 1979, Sir Magdi Yacoub developed the Remodeling technique which involves sinus segment replacement with Dacron graft and coronary button reimplantation. In recent iterations of the remodeling operation, aortic annuloplasty has been shown to prevent annular dilation and is considered when the annulus is greater than 25 mm (3).
In 1988, Dr. Tirone David performed a VSRR for a patient with a dilated root and normal aortic valve function. The described reimplantation technique has the advantage of annular stabilization whereby the entire aortic valve complex, including the annulus is ultimately supported within the Dacron replacement graft (4,5). This is particularly important when treating patients with a connective tissue disorder who have annuloaortic ectasia.
The reimplantation technique is notable for outstanding durability, with a majority of patients requiring no aortic valve re-intervention beyond twenty years. Critical components for successful operative and long-term outcomes include careful and appropriate patient selection, thorough understanding of aortic root anatomy and geometry, and meticulous surgical technique with no residual aortic insufficiency (AI).
Operative technique
Preoperative imaging and valve assessment
All patients should be evaluated with standard preoperative testing including an electrocardiogram, echocardiogram, coronary angiogram, and computed tomography (CT) with contrast. Echocardiogram imaging is crucial to assess aortic root and valve anatomy to determine valve sparability. In general, our preference is to obtain a transesophageal echocardiogram (TEE) as part of the preoperative work-up for high quality and accurate images.
There are two TEE views which are particularly informative when assessing valve sparability. While the long axis view allows measurement of aortic root geometry as well as cusp opening and closing, its most important contribution is assessing the degree of AI and jet centricity. A central jet of any degree implies cusp symmetry and failure of cusp coaptation as the etiology of valve regurgitation and suggests that the valve can be spared irrespective of the degree of preoperative AI, provided there is no cusp degeneration. An eccentric jet of AI implies cusp asymmetry and suggests that cusp repair along with correction of aortic root geometry will be required to correct valvular regurgitation. An eccentric AI jet can be due to a restricted cusp or a prolapsed cusp (with or without free margin elongation). In the scenario of cusp prolapse, aortic valve cusps can typically be repaired if there is elongation of the free margin which can be shortened by plication. Of note in bicuspid valve anatomy, cusp prolapse of the conjoined cusp is invariably associated with free margin elongation and it is our opinion that almost any bicuspid valve can be repaired, irrespective of the degree of preoperative AI, in the absence of cusp degeneration.
The short axis on TEE without color flow Doppler provides valuable information regarding the cusp opening and closing mechanisms during the cardiac cycle, degree of cusp degeneration and symmetry. Echogenic irregularity of the cusp free margins suggests cusp degeneration; other anatomic variables which are assessed are symmetry and restriction of cusp movement. It is important to keep in mind that TEE assessment of valve anatomy is user dependent and the final determination of valve sparability is direct visual inspection.
Exposure and cannulation
A median sternotomy is performed, and arterial cannulation can be central or peripheral depending on whether circulatory arrest is required as part of the aortic repair and the specific type of circulation management being employed. Venous cannulation is typically central via the right atrium using a dual-stage venous cannula and a left ventricular vent is also placed (Figure 1). Cardioplegia is typically performed with antegrade cardioplegia in combination with retrograde cardioplegia according to surgeon preference. Following initiation of cardiopulmonary bypass, aortic cross clamping and cardiac arrest, the aortic root is exposed via transverse aortotomy performed 2 cm above the sinotubular junction. One retraction suture is placed in the aorta.
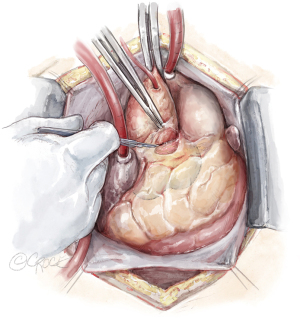
Dissection of the aortic root and creation of the coronary buttons
The root is mobilized from the surrounding cardiac structures; dissection is performed in a methodical manner. Every cardiac structure is at risk for injury during this process. Stay sutures using 4-0 polypropylene sutures are placed at the top of each commissural post to enhance exposure. These same sutures will then be used to tack the commissural posts inside the Dacron replacement graft. During this portion of the operation, the assistant should be using two fine graspers for retraction.
The non-coronary sinus tissue is typically excised first which allows the root to open like a flower blooming. Next the left sinus is excised, and the left main (LM) coronary button is created. Metzenbaum scissors are used with the tips pointed away from the coronary ostia to excise each sinus and create the coronary buttons. Lastly, the right sinus is excised, and the right coronary button is created. “Jehle” cardioplegia catheters are directed into the left and right coronary ostium and affixed with a vessel loop. These catheters are atraumatic and our preferred choice for continuous antegrade delivery of cardioplegia. A polypropylene suture is placed at the top of each button for retraction. It is important to note that the left button only requires mobilization for a few millimeters as this will be sufficient length for reattachment whereas the right may require more extensive dissection to avoid kinking during button reimplantation (Figure 2).
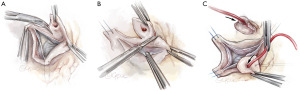
The rim of aortic tissue left attached to the annulus should be around 4–5 mm. Complete mobilization of the entire aortic root complex to the sub-annular level is essential for successful VSRR reconstruction. Our preferred technique for complete mobilization is similar to the method popularized by the Brussels group with a 360-degree dissection using electrocautery down to the level of the virtual basal ring (6)
Valve inspection and assessment for sparability
When examining the cusp anatomy for valve competency, significant calcification, cusp degeneration and excessive fenestrations represent contraindications to performing a VSRR procedure.
To assess valve competency, upward traction is created by pulling up on the commissural post stitches in order to mimic the distended state of the root. The left ventricular vent suction is maximized to create a vacuum in the left ventricle (LV) to induce cusp coaptation and is known as the “suction test”. In general adequacy of cusp coaptation length and any degree of prolapse are noted. The presence of prolapse can be corrected if cusp free margin is elongated. If prolapse is occurring in the setting of a shortened free margin, VSRR is aborted. Understanding the anatomic features of cusp anatomy and coaptation which correlates with valve competency is absolutely critical for optimal outcomes (Figure 3).
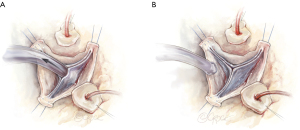
Graft sizing
Graft sizing for a trileaflet valve is performed using a modified version of the Feindel-David formula which is [(avg cusp height × 4/3)+ 8–10 mm]. We prefer a straight Dacron graft which is felt to provide more flexibility in recreating aortic root geometry. For BAV anatomy, graft sizing is loosely based on the free margin of the reference cusp, minus approximately 4–6 mm, which yields the optimal graft size (Figure 4).
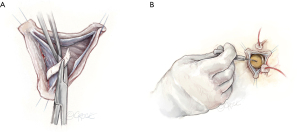
Placement of sub-annular stitches
Six annular stitches are placed in total: one at the nadir of each cusp and one beneath each commissure. The only exception is at the right/non commissure where we place a suture outside the commissural post along the right atrial tissue to avoid injuring the membranous septum and conduction system (Figure 5).
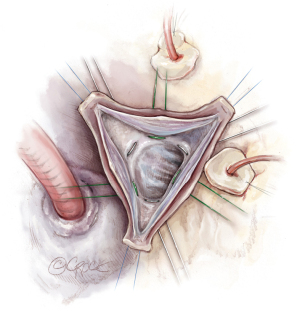
Tacking the commissural posts
This represents a critical portion of the operation because it effectively sets the geometry of the aortic root into its permanent configuration. From this point forward, any correction of AI will involve cusp repair. Thus, before tying the commissural post sutures, it is essential to ensure that the overall geometry is satisfactory. Once the commissural posts are tacked inside the graft, the suction test is performed to assess valve coaptation as described above (Figure 6). It should be noted that the heights and angles of the posts can be adjusted to optimize valve competency and the heights of each post are not necessarily equivalent. When the optimal geometry is achieved, the commissural sutures are tied on the outside of the graft.
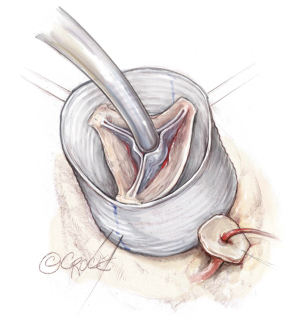
Valve reimplantation
A running hemostatic suture line is created for all three sinuses and is achieved by starting at the nadir of each sinus and suturing towards the top of each corresponding commissural post. The typical order is left, right and non-coronary sinuses. In order to minimize the risk of bleeding, several additional horizontal mattress sutures are placed along each cusp’s suture line. This step only takes a few additional minutes and reduces bleeding after cross-clamp removal (Figure 7).
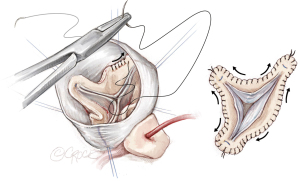
Cusp repair and correction of prolapse
After the valve has been reimplanted and the hemostatic suture line completed, cusp repair is performed prior to coronary button reimplantation. By performing cusp repair at this stage of the procedure, one avoids any torque on the graft and distortion of root geometry which may occur as a result of the coronary buttons being attached. Cusp prolapse in the setting of an elongated free margin is corrected by plication using 5-0 or 6-0 polypropylene suture (Figure 8).
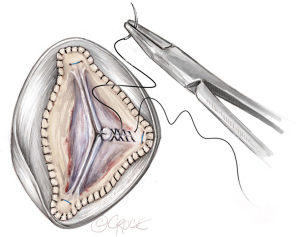
Coronary button reimplantation
The LM artery is typically reimplanted first followed by the right coronary artery (RCA). The precise location is achieved by laying the graft against the LM ostium posteriorly and an ophthalmic cautery is used to create an opening in the left neo-sinus for the left button. The left button is typically reimplanted in the lower part of the left neo-sinus. The entire suture posterior line is done in a two-bite step and is performed primarily backhand while the anterior suture line can be done forehand in one bite depending on the geometry.
For the right coronary button, upward traction is placed on both the graft and the artery to determine the optimal location for button placement. Typically, the RCA is reimplanted higher on the right neo-sinus to avoid kinking of the vessel, compromise of flow, and subsequent right ventricular malperfusion. The RCA is sutured in a similar manner as the LM button (Figure 9).
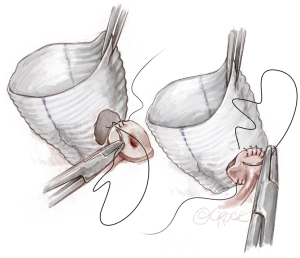
Last steps
Following completion of the distal anastomosis, release of the cross clamp and coming off cardiopulmonary bypass, TEE is used to cardiac function and valve competency.
Commentary
Optimal long-term clinical outcomes for performing VSRR using the reimplantation technique are dependent on three key components: careful and appropriate patient selection, thorough understanding of aortic root anatomy and geometry, meticulous surgical technique and leaving the operating room without AI.
Appropriate patients for this operation are those with minimal co-morbidities who have at least twenty years of life expectancy. In addition, these patients require appropriate valve anatomy for VSRR including normal cusp anatomy with minimal degeneration for optimal clinical success.
When performed on patients with suitable anatomy, long term outcomes for VSRR are outstanding. In 2014, David et al. published outcomes chronicling a quarter of a century of experience in 371 consecutive patients who underwent VSRR from 1988–2010. A total of 296 patients underwent a reimplantation technique and 75 underwent remodeling, with a mean age of forty-seven. Reimplantation was associated with a 4% risk of recurrent AI (moderate or severe, 12/296) and 95% freedom from re-operation at eighteen years (7).
Similarly, we have been able to demonstrate excellent long-term durability with the reimplantation technique in more complex clinical scenarios including type A dissection, preoperative AI, and bicuspid valve anatomy. The following studies outline our experience with data derived from a single institutional aortic database. In a report of fifty-seven patients undergoing VSRR in the setting of acute type A dissection, 30-day mortality was found to be 2%, and at nine years only one patient required valve reintervention (8).
In 2017, a comparison of VSRR was performed in patients who had preoperative >2+ AI vs. ≤2+ AI. Operative mortality was 2.2% and similar between groups. Patients with preoperative AI >2+ experienced significant reverse LV remodeling and improvements in LV function. In addition, eight-year freedom from recurrent AI >2+ was not impacted by the degree of preoperative AI and similar between groups (89.1% vs. 92.7%, P=0.57) (9).
When examining the impact of preoperative jet centricity in patients with >2+ preoperative AI, preoperative eccentric AI did not negatively impact operative outcomes when compared to preoperative central AI (30 d mortality 0% vs. 4%, eccentric vs. concentric, P=0.64). When comparing long-term outcomes, ten-year cumulative incidence of aortic valve replacement (AVR) (93% vs. 100%, P=0.98) and ten-year freedom from >1+ AI (15% vs. 0%, P=0.97) were similar between patients with preoperative eccentric AI compared to those with preoperative central AI (10).
Lastly, when performed in the setting of BAV anatomy, irrespective of the degree of preoperative AI, VSRR was found to have outstanding operative outcomes and long-term freedom from recurrent AI and AVR (11). When compared to trileaflet valves, performing VSRR in the setting of BAV anatomy resulted in equivalent operative and long-term outcomes, with cumulative incidence of >2+ AI 2% and AVR 4.3% (P=0.75) for trileaflet valve patients and 7.7% and 7.7% (P=0.81) for bicuspid valve patients at five years follow up (12).
In summary, VSRR using the reimplantation technique is a safe and durable operation when performed in a variety of clinical scenarios.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Cameron D. Valve-sparing aortic root replacement. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2012;15:20-3. [Crossref] [PubMed]
- David TE. Aortic valve sparing operations. Semin Thorac Cardiovasc Surg 2011;23:146-8. [Crossref] [PubMed]
- Bilkhu R, Tome M, Marciniak A, et al. Does the Aortic Annulus Dilate After Aortic Root Remodeling? Ann Thorac Surg 2020;110:943-7. [Crossref] [PubMed]
- David TE. The aortic valve-sparing operation. J Thorac Cardiovasc Surg 2011;141:613-5. [Crossref] [PubMed]
- Jahanyar J, de Kerchove L, El Khoury G. Bicuspid aortic valve repair: the 180°-Reimplantation technique. Ann Cardiothorac Surg 2022;11:473-81. [Crossref] [PubMed]
- David TE, Feindel CM, David CM, et al. A quarter of a century of experience with aortic valve-sparing operations. J Thorac Cardiovasc Surg 2014;148:872-9; discussion 879-80. [Crossref] [PubMed]
- Rosenblum JM, Leshnower BG, Moon RC, et al. Durability and safety of David V valve-sparing root replacement in acute type A aortic dissection. J Thorac Cardiovasc Surg 2019;157:14-23.e1. [Crossref] [PubMed]
- Keeling WB, Leshnower BG, Binongo J, et al. Severity of Preoperative Aortic Regurgitation Does Not Impact Valve Durability of Aortic Valve Repair Following the David V Valve Sparing Aortic Root Replacement. Ann Thorac Surg 2017;103:756-63. [Crossref] [PubMed]
- Deas DS Jr, Lou X, Leshnower BG, et al. Preoperative Eccentric Aortic Regurgitation and Outcomes Following Valve-Sparing Root Replacement. Semin Thorac Cardiovasc Surg 2021;33:627-34. [Crossref] [PubMed]
- Kayatta MO, Leshnower BG, McPherson L, et al. Valve-Sparing Root Replacement Provides Excellent Midterm Outcomes for Bicuspid Valve Aortopathy. Ann Thorac Surg 2019;107:499-504. [Crossref] [PubMed]
- Kayatta MO, Leshnower BG, McPherson L, et al. Valve Sparing Root Replacement Provides Similar Midterm Outcomes in Bicuspid and Trileaflet Valves. Ann Thorac Surg 2019;107:54-60. [Crossref] [PubMed]






