Prophylactic cerebrospinal fluid drainage and spinal cord ischemia in thoracic and thoracoabdominal endovascular procedures: a systematic review and meta-analysis
Introduction
Spinal cord ischemia (SCI) is one of the most devastating complications of thoracic aortic procedures. The traditional standard of open thoracic aortic repair sees SCI rates reported between 5–21% (1). Thoracic endovascular aortic repair (TEVAR) sees lower SCI rates reported between 0–17%, with procedures on thoracoabdominal aortic aneurysms (TAAA) carrying the highest risk (2-4).
Such procedures carry an inherent risk of spinal cord injury as thoracic aortic pathologies often involve branches of the aorta directly involved in the vascular supply of the spinal cord. The artery of Adamkiewicz had been traditionally thought to be the major culprit of such malperfusion but empirical experience has shown that occlusion of collateral supply (intercostal, lumbar, subclavian arteries) may contribute a larger factor to SCI, and incidence of SCI increases with extent of aortic coverage (5,6).
The perfusion of these critical arteries is termed spinal cord perfusion pressure (SCPP), and many any neuroprotective techniques have since been employed to maintain SCPP and reduce the risk of paraplegia. Effective surgical techniques include left subclavian artery revascularization and the use of fenestrated and branched endografts (7,8). Similarly, anesthesiology techniques include mean arterial pressure (MAP) maintenance, cardiac index maintenance, and monitoring of motor and sensory evoked potentials throughout the procedure (9). One debated method of maintaining SCPP is through cerebrospinal fluid drainage (CSFD), for which the body of literature remains divided. Various studies have reported reduced risks of SCI following CSFD, and patients who receive routine prophylactic CSFD may receive the greatest benefit (10-13). Other studies suggest that SCI rates remain similar between drained and non-drained patients (14,15). As such, the use of CSFD has remained stable around 30% over the past decade (16).
Furthermore, CSFD is not a benign procedure, and carries complications including that of paraplegia itself. Such complications have been reported up to rates of 10% and multiple centers have abandoned the neuroprotective procedure following severe adverse outcomes, leading to many clinicians warning against overuse of the procedure (17-20).
This systematic review and meta-analysis address the various CSFD indications implemented in TEVAR procedures for different aortic pathologies to provide more insight to the global experience of CSFD and its efficacy in protecting against SCI.
Methods
Literature search strategy
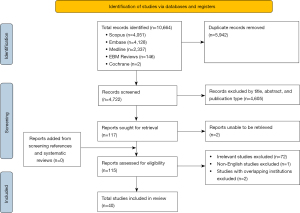
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Figure 1) recommendations (21). Scopus, Embase, Medline, Cochrane and Evidence-Based Medicine (EBM) Reviews databases were searched by two independent authors (CHJ Chen, H Jiang) for the following electronic keyword and medical subject heading (MeSH) terms: (“TEVAR” OR “EVAR” OR “endovascular”) AND (“aneurysm” OR “aortic dissection” OR “TAA” OR “TAAA” OR “thoracic” OR “thoracoabdominal”) AND ((“spinal cord ischemia” OR “spinal cord injury” OR “SCI” OR “paraplegia” OR “weakness” OR “paresis”) OR (“cerebrospinal fluid” OR “CSF” OR “CSFD” OR “drain” OR “drainage”)). Studies published between the inception of the database to September 2022 containing the search terms in the title and abstract were included for screening following removal of duplicate studies. Published systematic reviews and references were manually screened for eligible studies.
Eligibility criteria
The inclusion criteria for the systematic review and meta-analysis include (I) studies with more than ten adult (>18 years of age) patients undergoing CSFD; (II) studies reporting SCI rates; and (III) English studies. Studies were excluded from analysis if it met any of the following exclusion criteria: (I) studies reporting open surgical procedures*; (II) studies focusing on redo endovascular procedures; and (III) editorials, reviews, conference abstracts and case reports. Studies were screened independently by two authors (CHJ Chen, VDD Nguyen) for inclusion in the meta-analysis and discrepancies were discussed with the third author (H Jiang).
Data extraction and critical appraisal
Data was extracted by three authors (CHJ Chen, H Jiang, VDD Nguyen) independently. Primary outcomes for this study included immediate, delayed, transient and permanent SCI. Secondary outcomes included CSFD complications, procedural complications, and mortality. Quality assessment was completed using a modified schema from the Institute of Health Economics (Alberta, Canada) (Table S1) (22). Studies were classified as low quality, moderate quality and high quality if it satisfied fewer than 10 criteria, 10–12 criteria and more than 12 criteria, respectively.
Statistical analysis
Pooled means and proportions were calculated using OpenMeta[Analyst] (Center for Evidence-based Medicine, Brown University, USA) (23). Continuous and binary Dersimonian-Laird random effects models were used for meta-analyses of means and proportions, respectively. Pooled means are presented as mean [95% confidence interval (CI)] and pooled proportions are presented as rate (95% CI). The Box-Cox method described by McGrath et al. was used to convert median and interquartile range to mean and standard deviation to facilitate pooling (24). Odds ratios (ORs) and 95% CI were calculated for data comparing CSFD and non-CSFD patients using Review Manager (RevMan) (Version 5.4, The Cochrane Collaboration, 2020) (25). Heterogeneity between studies was calculated using the I2 statistic, with I2 values of 0–49%, 50–74% and 75–100% representing low, moderate and high heterogeneity, respectively. P values <0.05 were considered statistically significant. Publication bias was assessed by visual inspection of funnel plots by two authors (CHJ Chen and H Jiang) independently. Significant asymmetry in funnel plots suggested publication bias for the outcome.
Results
Study details
A total of 10,664 records were identified following an extensive literature search, of which 40 studies (4,793 patients) were included following exclusion (Table 1). The earliest study was published in 2005. The majority of the data was sourced from the United States (16 studies), followed by Japan (5 studies), Germany (4 studies), and Italy (4 studies) (Table 1). Seventeen studies were found to be of low quality, 21 of medium quality, and two studies were of high quality. There was a total of 14 comparative studies and 26 single arm studies included (Table 2).
Table 1
| First author, publication year | Data type | Data source | Country |
|---|---|---|---|
| Acher (26), 2016 | Single center | University of Wisconsin School of Medicine and Public Health | United States |
| Adams (27), 2019 | Single center | Carilion Roanoke Memorial Hospital | United States |
| Addas (28), 2022 | Single center | University Health Network Research Centre, Toronto | Canada |
| Angiletta (29), 2021 | Multicenter | University of Bari School of Medicine; University of Insubria School of Medicine; Bolzano Hospital; University of Padua School of Medicine | Italy |
| Arnaoutakis (30), 2014 | Single center | Johns Hopkins Hospital | United States |
| Banno (31), 2021 | Single center | Nagoya University Graduate School of Medicine | Japan |
| Bisdas (18), 2015 | Single center | St. Franziskus Hospital | Germany |
| Bobadilla (32), 2013 | Single center | St. Claire Health Centre, Kentucky | United States |
| Chaudhary (33), 2021 | Single center | Beth Israel Deaconess Medical Center, Harvard Medical School | United States |
| Cheung (34), 2005 | Single center | University of Pennsylvania | United States |
| Chuter (35), 2008 | Single center | University of California, San Francisco | United States |
| D'Oria (36), 2019 | Single center | University Hospital of Cattinara ASUITs, Trieste | Italy |
| D'Souza (37), 2009 | Single center | Mayo Clinic, Rochester, Minnesota | United States |
| Desart (38), 2013 | Single center | University of Florida, Gainesville | United States |
| Fossaceca (39), 2013 | Single center | Maggiore Della Carita Hospital, A. Avogadro University, Novara | Italy |
| Hiraoka (40), 2018 | Single center | Kurashiki Central Hospital, Kurashiki, Okayama | Japan |
| Hnath (41), 2008 | Single center | Albany Medical Center, New York | United States |
| Iafrancesco (42), 2014 | Single center | Queen Elizabeth University Hospital | United Kingdom |
| Iyer (43), 2006 | Single center | McGill University | Canada |
| Juszczak (19), 2019 | Single center | Heartlands Hospital, Birmingham | United Kingdom |
| Kato (44), 2015 | Single center | Morinomiya Hospital | Japan |
| Khoynezhad (45), 2013 | Multicenter | 20 different centers (RESCUE trial)* | United States |
| Kitpanit (46), 2021 | Single center | New York Presbytarian Hospital | United States |
| Kotelis (47), 2015 | Single center | Heidelberg University Hospital | Germany |
| Maier (48), 2019 | Single center | University Heart Center Freiburg | Germany |
| Maurel (49), 2015 | Single center | CHRU de Lille | France |
| Mazzeffi (50), 2018 | Single center | University of Maryland | United States |
| Nathan (51), 2015 | Single center | University of Washington | United States |
| Pasqualucci (52), 2020 | Multicenter | Santa Maria della Misericordia University Hospital, Italy; Rashid Hospital, DHA, Dubai | Italy; United Arab Emirates |
| Preventza (53), 2009 | Single center | Arizona Heart Institute | United States |
| Rizk (54), 2021 | Single center | Ain Shams University, Cairo | Egypt |
| Schurink (55), 2007 | Single center | University Hospital Maastricht | Netherlands |
| Seike (15), 2022 | Single center | National Cerebral and Cardiovascular Center | Japan |
| Song (12), 2017 | Single center | Gangnam Severance Hospital, Yonsei University College of Medicine | South Korea |
| Sugiyama (56), 2022 | Single center | Shinshu University Hospital | Japan |
| Sulzinski (57), 2022 | Single center | Medstar Hospital, Washington | United States |
| Verma (58), 2022 | Single center | All India Institute of Medical Sciences | India |
| Verzini (59), 2020 | Single center | AOU Citta della Salute e della Scienza, University of Turin; S Giovanni-Addolorata Hospital, Rome; A.O. Perugia, Perugia | Italy |
| Yang (60), 2019 | Single center | University of British Columbia | Canada |
| Zipfel (61), 2013 | Single center | Deutsches Herzzentrum Berlin | Germany |
*, the individual centers in the RESCUE trial in the study by Khoynezhad et al. 2013 were examined and found to not overlap with the other studies in the included.
Table 2
| First author, publication year | Patient recruitment | Data source type | Data type (comparison) | Years of recruitment | Patients (n) | Quality of evidence |
|---|---|---|---|---|---|---|
| Acher (26), 2016 | Retrospective | Single center | Single arm | 2005–2014 | 155 | Low |
| Adams (27), 2019 | Retrospective | Single center | Multi-arm (Gore TAG endoprosthesis post-FDA approval vs. phase II trial) | 2005–2006 | 50 | Medium |
| Addas (28), 2022 | Retrospective | Single center | Single arm | 2017–2020 | 17 | Medium |
| Angiletta (29), 2021 | Retrospective | Multicenter | Single arm | 2018–2019 | 14 | High |
| Arnaoutakis (30), 2014 | Retrospective | Single center | Multi-arm (adjunctive procedure vs. no adjunctive procedure for TEVAR) | 2005–2012 | 90 | Low |
| Banno (31), 2021 | Retrospective | Single center | Multi-arm (SCI vs. no SCI) | 2008–2018 | 212 | Medium |
| Bisdas (18), 2015 | Retrospective | Single center | Multi-arm (SCI vs. no SCI) | 2010–2014 | 142 | Medium |
| Bobadilla (32), 2013 | Retrospective | Single center | Single arm | 2005–2012 | 94 | Low |
| Chaudhary (33), 2021 | Retrospective | Single center | Multi-arm (CSFD vs. no CSFD) | 2014–2019 | 235 | Low |
| Cheung (34), 2005 | Prospective | Single center | Single arm | 1999–2004 | 75 | Medium |
| Chuter (35), 2008 | Prospective | Single center | Single arm | 2006–2007 | 22 | Medium |
| D'Oria (36), 2019 | Retrospective | Single center | Single arm | 2015–2017 | 24 | Medium |
| D'Souza (37), 2009 | Retrospective | Single center | Single arm | 2001–2007 | 20 | Low |
| Desart (38), 2013 | Retrospective | Single center | Multi-arm (SCI vs. no SCI) | 2000–2011 | 607 | Medium |
| Fossaceca (39), 2013 | Retrospective | Single center | Single arm | 2005–2011 | 53 | Low |
| Hiraoka (40), 2018 | Retrospective | Single center | Multi-arm (SCI vs. no SCI) | 2008–2014 | 175 | Medium |
| Hnath (41), 2008 | Prospective | Single center | Multi-arm (CSFD vs. no CSFD) | 2004–2006 | 121 | Medium |
| Iafrancesco (42), 2014 | Retrospective | Single center | Single arm | 2007–2012 | 62 | Low |
| Iyer (43), 2006 | Retrospective | Single center | Multi-arm (elective vs. emergent) | 1999–2005 | 70 | Low |
| Juszczak (19), 2019 | Retrospective | Single center | Single arm | 2008–2017 | 270 | Medium |
| Kato (44), 2015 | Retrospective | Single center | Single arm | 2007–2014 | 54 | Low |
| Khoynezhad (45), 2013 | Prospective | Multicenter | Single arm | 2010–2012 | 59 | Medium |
| Kitpanit (46), 2021 | Prospective | Single center | Multi-arm (SCI vs. no SCI) | 2014–2019 | 106 | Medium |
| Kotelis (47), 2015 | Prospective | Single center | Single arm | 2012–2013 | 30 | Medium |
| Maier (48), 2019 | Retrospective | Single center | Multi-arm (CSFD vs. no CSFD) | 1998–2014 | 223 | Low |
| Maurel (49), 2015 | Retrospective | Single center | Multi-arm (before vs. after implantation of modified peri-operative protocol) | 2004–2013 | 204 | Medium |
| Mazzeffi (50), 2018 | Retrospective | Single center | Single arm | 2011–2015 | 102 | Low |
| Nathan (51), 2015 | Retrospective | Single center | Single arm | 2006–2013 | 47 | Medium |
| Pasqualucci (52), 2020 | Prospective | Multicenter | Single arm | 2016–2018 | 47 | Low |
| Preventza (53), 2009 | Prospective | Single center | Single arm | 2000–2008 | 346 | Low |
| Rizk (54), 2021 | Retrospective | Single center | Single arm | 2014–2020 | 23 | Low |
| Schurink (55), 2007 | Retrospective | Single center | Single Arm | 2000–2005 | 13 | Low |
| Seike (15), 2022 | Retrospective | Single center | Multi-arm (CSFD vs. no CSFD) | 2009–2020 | 204 | Medium |
| Song (12), 2017 | Prospective | Single center | Single arm | 2012–2014 | 81 | Medium |
| Sugiyama (56), 2022 | Retrospective | Single center | Single arm | 2011–2019 | 31 | Medium |
| Sulzinski (57), 2022 | Retrospective | Single center | Single arm | 2017–2018 | 130 | Low |
| Verma (58), 2022 | Retrospective | Single center | Multi-arm (stent graft length ≤200 mm vs. stent graft length >200 mm) | 2014–2020 | 38 | Medium |
| Verzini (59), 2020 | Retrospective | Single center | Single arm | 2012–2018 | 21 | High |
| Yang (60), 2019 | Retrospective | Single center | Single arm | 2007–2016 | 130 | Medium |
| Zipfel (61), 2013 | Retrospective | Single center | Single arm | 2000–2010 | 406 | Low |
n, number; FDA, Food and Drug Association; TEVAR, thoracic endovascular aortic repair; SCI, spinal cord ischemia; CSFD, cerebrospinal fluid drainage.
Procedures
All studies included in the meta-analysis targeted thoracic aortic pathologies. Most studies analyzed multiple aortic pathologies, with the most common pathology being thoracic and thoracoabdominal aneurysmal disease. Standard TEVAR was studied in 34 studies, with 11 studies exclusively or simultaneously addressing fenestrated or branched endograft procedures (Table S1). Elective, urgent, and emergent procedures were all included in the study. Selective CSFD protocols were utilized in 23 studies and routine in 17 studies. Where reported, brief details about the selective indication of each study are included in Table S2.
Baseline characteristics
A total of 4,793 patients undergoing thoracic endovascular aortic procedures from 40 studies were included in the meta-analysis. The mean age was 68.8 years (95% CI: 67.3–70.3; I2=99%). Of these patients, 70.9% were male (95% CI: 66.7–75.0%; I2=90%). The left subclavian artery (LSA) was covered in 42.7% of patients (95% CI: 32.3–53.2%; I2=98%), and 18.7% (95% CI: 14.2–23.1%; I2=94%) of the total number of patients underwent a LSA revascularization procedure prior to their operation; 30.6% (95% CI: 23.5–37.7%; I2=98%) of patients had prior aortic repair, with 19.3% (95% CI: 14.0–24.6%; I2=97%) having had prior abdominal aortic repair, and 18.8% (95% CI: 12.9–24.8%; I2=97%) having had prior thoracic aortic repair (Table 3). Further patient comorbidities and vascular risk factors are detailed in Table 3. Baseline characteristics were highly heterogeneous throughout the various patient cohorts, attributable to the variety of conditions requiring TEVAR and their associated risk factors. There was a mixture of studies focusing on a single disease process, such as aneurysmal disease or blunt aortic injury, and also studies which covered multiple disease processes (Table S1).
Table 3
| Characteristic | Patients (n) [studies] | Weighted pooled estimate (95% CI) | Heterogeneity I2 (%) |
|---|---|---|---|
| Age (years)* | 4,624 [37] | 68.8 (67.3, 70.3) | 99 |
| Male (%) | 3,256 [38] | 70.9 (66.7, 75.0) | 90 |
| LSA coverage (%) | 1,234 [22] | 42.7 (32.3, 53.2) | 98 |
| LSA revascularization | 566 [24] | 18.7 (14.2, 23.1) | 94 |
| Prior aortic repair (%) | |||
| Any prior aortic repair | 1,047 [26] | 30.6 (23.5, 37.7) | 98 |
| Prior AAA repair | 644 [22] | 19.3 (14.0, 24.6) | 97 |
| Prior thoracic aneurysm repair | 295 [13] | 18.8 (12.9, 24.8) | 97 |
| Chronic renal insufficiency (%) | |||
| GFR >15 and not on hemodialysis* | 598 [21] | 19.6 (14.6, 24.5) | 94 |
| GFR <15 or on hemodialysis | 66 [13] | 3.7 (2.1, 5.3) | 60 |
| Hypertension (%) | 2,791 [30] | 79.0 (72.1, 85.8) | 97 |
| Dyslipidemia (%) | 825 [14] | 49.1 (35.5, 62.7) | 98 |
| Smoking history (%) | |||
| Any smoking history | 1,035 [19] | 51.3 (38.9, 63.7) | 98 |
| Current smoker | 272 [10] | 27.3 (19.0, 35.5) | 92 |
| History of COPD (%) | 888 [27] | 26.5 (21.2, 31.9) | 94 |
| History of CAD (%) | 911 [28] | 28.8 (22.9, 34.6) | 96 |
| History of CHF (%) | 79 [10] | 5.4 (3.0, 7.9) | 79 |
| History of PAD (%) | 180 [10] | 15.4 (9.9, 20.9) | 94 |
| History of DM (%) | 398 [25] | 14.2 (10.8, 17.6) | 89 |
| History of stroke or CVD (%) | 243 [14] | 11.2 (7.5, 14.9) | 90 |
*, Khoynezhad et al. 2013 was excluded following sensitivity analysis. Chronic renal insufficiency was defined as a GFR less than 60 mL/min or a creatinine greater than 1.5 mg/dL. Khoynezhad et al. 2013 was excluded from sensitivity analysis as its young patient cohort significantly skewed the mean age. This may be attributable to the mode of injury (BAI) leading to TEVAR in his patient population. n, number of patients; CI, confidence interval; LSA, left subclavian artery; AAA, abdominal aortic aneurysm; GFR, glomerular filtration rate; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; CHF, congestive heart failure; PAD, peripheral artery disease; DM, diabetes mellitus, CVD, cerebrovascular disease; BAI, blunt aortic injury.
SCI rates
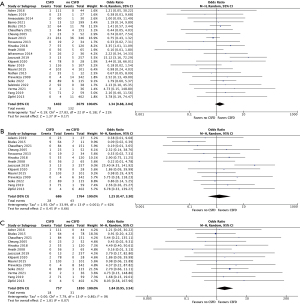
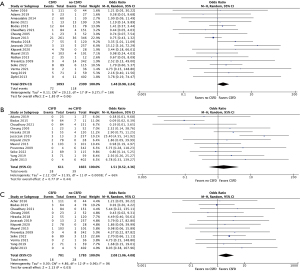
The incidence of SCI in this study was 232, translating to a rate of 3.5% (95% CI: 2.6–4.4%; I2=67%) (Figure 2). The immediate SCI rate, defined as presence at the emergence of anesthesia, was 1.3% (95% CI: 0.7–1.8%; I2=60%), and the delayed SCI rate was 1.9% (95% CI: 1.2–2.5%; I2=53%). Compared to non-drained patients, CSFD patients demonstrated no significant difference in rates of any SCI (OR 1.34; 95% CI: 0.88–2.04; P=0.17), transient SCI (OR 1.84; 95% CI: 0.95–3.54; P=0.07) or permanent SCI (OR 1.25; 95% CI: 0.47–3.30; P=0.66). Routine CSFD also did not produce any significant difference in rates of any SCI (OR 0.54; 95% CI: 0.14–2.03; P=0.36), transient SCI (OR 0.16; 95% CI: 0.01–3.13; P=0.23) and permanent SCI (OR 0.27; 95% CI: 0.03–2.40; P=0.24). Selective CSFD produced comparable results for rates of any SCI (OR 1.48; 95% CI: 0.98–2.24; P=0.06) and permanent SCI (OR 1.51; 95% CI: 0.52–4.36; P=0.44), but this population was associated with an increased rate of transient SCI (OR 2.08; 95% CI: 1.06–4.08; P=0.03). CSFD failed to produce any significant effect on SCI rate in the population with aneurysmal disease (OR 1.39; 95% CI: 0.81–2.37; P=0.23) or dissection related disease (OR 1.31; 95% CI: 0.17–9.87; P=0.79). There was a trend towards an increased rate of SCI in drained elective patients (OR 2.51; 95% CI: 0.97–6.52; P=0.06), but this result did not reach significance (Table 4; Figures 3-6).
Table 4
| Outcome | Patients* (n) [studies] | Odds ratio (95% CI) | P value | Heterogeneity I2 (%) |
|---|---|---|---|---|
| CSFD vs. non-CSFD | ||||
| Any SCI | 102 [40] | 1.34 (0.88, 2.04) | 0.17 | 21 |
| Transient SCI | 33 [32] | 1.84 (0.95, 3.54) | 0.07 | 0 |
| Permanent SCI | 36 [32] | 1.25 (0.47, 3.30) | 0.66 | 62 |
| Routine CSFD vs. non-CSFD | ||||
| Any SCI | 28 [19] | 0.54 (0.14, 2.03) | 0.36 | 28 |
| Transient SCI | 12 [14] | 0.16 (0.01, 3.13) | 0.23 | – |
| Permanent SCI | 5 [14] | 0.27 (0.03, 2.40) | 0.24 | 0 |
| Selective CSFD vs. non-CSFD | ||||
| Any SCI | 78 [23] | 1.48 (0.98, 2.24) | 0.06 | 16 |
| Transient SCI | 21 [19] | 2.08 (1.06, 4.08) | 0.03 | 0 |
| Permanent SCI | 31 [19] | 1.51 (0.52, 4.36) | 0.44 | 66 |
| TAA and TAAA only (CSFD vs. non-CSFD) | ||||
| Any SCI | 33 [8] | 1.39 (0.81, 2.37) | 0.23 | 0 |
| Elective procedures only (CSFD vs. non-CSFD) | ||||
| Any SCI | 26 [5] | 2.51 (0.97, 6.52) | 0.06 | 46 |
*, refers to patients of each category who received CSFD prior to TEVAR and experienced SCI. n, number of patients; CI, confidence interval; CSFD, cerebrospinal fluid drainage; SCI, spinal cord ischemia; TAA, thoracic aortic aneurysm; TAAA, thoracoabdominal aortic aneurysm.


Publication bias was assessed for data comparing CSFD and non-CSFD patients. There was no convincing evidence of funnel plot asymmetry on visual inspection for any SCI, permanent SCI or transient SCI in patients undergoing CSFD versus non-CSFD patients (Figures S1-S3).
CSFD complication rates
A spinal headache was reported in 4.3% (95% CI: 1.8–6.9%; I2=73%) of patients undergoing CSFD procedures. Major complications were reported in 1.6% (95% CI: 0.8–2.4%; I2=22%) of CSFD procedures. These complications included meningitis in 0.6% (95% CI: 0.2–1.1%; I2=0) of patients, a CSF leak requiring reintervention in 0.7% (95% CI: 0.2–1.1%; I2=0), frank insertion site bleeding in 0.7% (95% CI: 0.2–1.1%; I2=0), a retained catheter tip in 0.7% (95% CI: 0.2–1.2%; I2=0), epidural or spinal hematoma in 0.9% (95% CI: 0.4–1.4%; I2=0), intracranial or subdural hemorrhage in 0.8% (95% CI: 0.3–1.3%; I2=0), significant paraparesis or paraplegia independent to the operation in 0.8% (95% CI: 0.3–1.3%; I2=0), and death in 0.6% (95% CI: 0.2–1.0%; I2=0) (Table 5).
Table 5
| CSFD related complication | Patients (n) [studies] | Weighted pooled estimate (95% CI), (%) | Heterogeneity I2 (%) |
|---|---|---|---|
| Common complications | |||
| Spinal headache | 38 [10] | 4.3 (1.8, 6.9) | 73 |
| Major complications* | |||
| Any major complication | 35 [19] | 1.6 (0.8, 2.4) | 22 |
| Meningitis | 1 [18] | 0.6 (0.2, 1.1) | 0.0 |
| CSF leak requiring intervention | 7 [17] | 0.7 (0.2, 1.1) | 0.0 |
| Frank insertion site bleeding | 6 [18] | 0.7 (0.2, 1.1) | 0.0 |
| Retained catheter tip | 3 [18] | 0.7 (0.2, 1.2) | 0.0 |
| Epidural or spinal hematoma | 8 [18] | 0.9 (0.4, 1.4) | 0.0 |
| Intracranial or subdural hemorrhage | 10 [19] | 0.8 (0.3, 1.3) | 0.0 |
| Significant paraparesis or paraplegia** | 6 [20] | 0.8 (0.3, 1.3) | 0.0 |
| Death | 2 [24] | 0.6 (0.2, 1.0) | 0.0 |
*, major complications are defined by events that may cause significant morbidity or that require repeat intervention. The major complications investigated in this study are meningitis, CSF leak requiring re-intervention, frank insertion site bleeding, retained catheter tip, epidural or spinal hematoma, intracranial or subdural hemorrhage, significant paraparesis or paraplegia and death. Spinal headaches and minor insertion site bleeding or bloody CSF are not accounted for in major complications. These complications are reported as directly related to the CSFD process, not as a result of the operation. **, significant paraparesis or paraplegia is defined as sensory change or weakness of the lower limbs that is prolonged or permanent. CSFD, cerebrospinal fluid drainage; n, number of patients; CI, confidence interval; CSF, cerebrospinal fluid.
Operative outcomes
In-hospital or perioperative mortality occurred at a rate of 1.7% (95% CI: 1.1–2.3%; I2=53%). Mid-term mortality, reported as mortality within a year, occurred at a rate of 4.5% (95% CI: 3.2–5.8%; I2=70%). Endoleaks of any type were reported at a rate of 12.9% (95% CI: 9.0–16.9%; I2=90%). Cerebrovascular accidents, defined as either stroke or transient ischemic attacks, occurred at a rate of 2.0% (95% CI: 1.3–2.7%; I2=38%). The mean reported total operation time was 180 minutes (range, 63–373 minutes), and the mean reported estimated blood loss was 187 mL (range, 50–714 mL) (Table 6).
Table 6
| Operative outcome | Patients (n) [studies] | Weighted pooled estimate (95% CI), (%) | Heterogeneity I2 (%) |
|---|---|---|---|
| In-hospital or perioperative mortality | 88 [31] | 1.7 (1.1, 2.3) | 53 |
| 30-day to 1-year mortality* | 176 [29] | 4.5 (3.2, 5.8) | 70 |
| Endoleak (of any type) | 246 [21] | 12.9 (9.0, 16.9) | 90 |
| Cerebrovascular accident (stroke or TIA) | 73 [26] | 2.0 (1.3, 2.7) | 38 |
*, Angiletta et al. 2021 was excluded following sensitivity analysis. Angiletta et al. was excluded from analysis following sensitivity analysis due to their high 30D-1Y mortality rate. n, number of patients; CI, confidence interval; TIA, transient ischemic attack; 30D-1Y, 30 days to 1 year.
Discussion
SCI is a major complication of aortic procedures that predisposes patients to notable life-long morbidity. While SCI rates have decreased following the widespread use of TEVAR in place of open aortic surgery, it continues to pose a significant threat to patients, occurring in 0–18% of patients (35,44). The current systematic review reported an overall SCI rate of 3.5%, including both transient and permanent SCI. TEVAR operators worldwide have adopted protocols aimed at monitoring and maintaining spinal cord perfusion during TEVAR, including neuromonitoring, intraoperative MAP maintenance and LSA revascularization (33,48,50,60). CSFD is a treatment adjunct that has been shown to reduce the risk of SCI in open aortic procedures. A randomized controlled trial (RCT) published in 2002 by Coselli and colleagues showed a significant decrease in paraplegia rates in CSFD patients undergoing open TAAA repair compared to their non-drained counterparts (62). Similarly, a systematic review and meta-analysis of RCTs and cohort studies showed significantly lower rates of paraplegia and paraparesis following CSFD in open aortic surgery (63).
The utility of CSFD in endovascular aortic procedures has been debated in the literature. Currently, there are no RCTs to the authors’ knowledge that investigates the benefit of CSFD in patients undergoing TEVAR. The current systematic review found no significant difference in either transient or permanent SCI rates between prophylactic CSFD and non-CSFD patients undergoing TEVAR for aortic aneurysms or dissections. This is consistent with a systematic review and meta-analysis by Wong et al., and we add that neither routine nor selective CSFD significantly reduced the risk of SCI (14). This contrasts the findings of a systematic review by Zhang et al., which found that routine CSFD is superior to selective CSFD in reducing the risk of SCI (11). However, no direct comparison was made between drained and non-drained patients in that study. Several cohort studies have reported a significant decrease in SCI rates with prophylactic CSFD. Maier et al. found a 3.9% decrease in SCI rates in patients undergoing CSFD compared to their non-drained counterparts, and Hnath et al. found an 8% decrease in SCI rates for the same comparison in 121 patients (41,48). Interestingly, we found an increased risk of transient SCI in patients who underwent selective CSFD compared to those who were not prophylactically drained. This may be due to the presence of preoperative risk factors for SCI in patients who are selected to undergo CSFD, such as extensive aortic coverage, prior aortic repair and distal descending aortic coverage (33,50,53).
Currently, CSFD protocols vary greatly between centers, and institutions report different CSF pressure targets and maximum drainage rates. As SCPP increases with lower spinal fluid pressures, lower CSF target pressures may decrease the incidence of SCI (32). In the current study, CSF pressure targets largely fall between 8 mmHg and 15 mmHg. Kato et al. and Maurel et al. adopted the most aggressive pressure targets of 7.3 mmHg (10 cmH2O) in their patient population (44,49). Previous studies have also found that greater drainage volumes were associated with more drain-related complications, requiring operators to set maximum drainage volumes or rates (64-66). Drainage rates generally range from 10 to 20 mL/hour in studies included in this systematic review. Kotelis et al. reported the highest mean drainage amount (714 mL) and the highest rate of drain-related complications (23%) of studies included in this meta-analysis (47). Further studies are required to determine the ideal pressure targets and drainage rates that strike a balance between optimal spinal cord protection with the least drain-related complications.
CSFD is associated with potential complications that may lead to significant long-term morbidity. A systematic review and meta-analysis of over 30 studies published by Rong et al. found an overall complication rate of 6.5% for patients undergoing CSFD for open and endovascular aortic procedures (67). The same study also reported a 2.5% rate of major complications, including epidural hematoma, intracranial hemorrhage, meningitis, and drain-related neurological deficit (67). This is similar to the current study, which reported a 1.6% risk of major complications following CSFD. The most common major complication in this systematic review was epidural or spinal hematoma (weighted pooled estimate of 0.9%), followed by intracranial or subdural hemorrhage (weighted pooled estimate of 0.8%) and significant paraparesis or paraplegia (weighted pooled estimate of 0.8%). Of these, intracranial hemorrhage is undoubtedly the most dangerous complication, which may lead to permanent neurological damage and even death despite immediate management. In the current study, both-drain-related deaths occurred following large intracranial bleeds, one intraoperatively and the other following drain removal (19,47). Several studies have found that a larger total volume drained was a significant risk factor for intracranial hemorrhage, thus requiring CSFD operators to take extra caution in monitoring total CSF drainage volume both intraoperatively and postoperatively (64-66). Such risks, in conjunction with debatable benefit of CSFD, should warn operators that CSFD prior to TEVAR should be a judicious decision and may vary patient to patient.
Limitations and future directions
Several limitations were present in the current study. The meta-analysis did not account for confounding variables that influence SCI risk, including procedural risk factors like increased thoracic aorta coverage and patient risk factors like previous abdominal aortic aneurysm (AAA) repair, peripheral artery disease, and renal insufficiency (46,68,69). Similarly, there was high heterogeneity in the baseline characteristics of patients included in this study which may have contributed to the overall SCI risk (Table 3). The CSFD protocol and other methods of reducing SCI risk varied greatly between studies, preventing an accurate comparison of the true impact of CSFD on SCI risk. Lastly, following quality analysis of the studies included, only two of 41 studies were deemed to be of high quality. Large, multicenter RCTs are required to further assess the utility of routine and selective CSFD in preventing SCI and to investigate true indications for selective prophylactic CSFD.
Conclusions
This study found no significant reduction in SCI rates in patients undergoing TEVAR with prophylactic CSFD. TEVAR teams need to stratify both the risk of SCI and CSFD complications when planning for endovascular intervention with prophylactic CSFD. Large RCTs are required to accurately assess the utility of routine and selective prophylactic CSFD in reducing SCI risk of TEVAR patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
*Studies reporting both open and endovascular procedures were included if data on patients undergoing endovascular procedures could be extracted independently.
References
- Gravereaux EC, Faries PL, Burks JA, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg 2001;34:997-1003. [Crossref] [PubMed]
- Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007;133:369-77. [Crossref] [PubMed]
- Malloy PC, Raghavan A, Elder T, et al. Cerebrospinal Fluid Drainage During Endovascular Aortic Aneurysm Repair: A Systematic Review of the Literature and Treatment Recommendations. Vasc Endovascular Surg 2020;54:205-13. [Crossref] [PubMed]
- Gaudino M, Khan FM, Rahouma M, et al. Spinal cord injury after open and endovascular repair of descending thoracic and thoracoabdominal aortic aneurysms: A meta-analysis. J Thorac Cardiovasc Surg 2022;163:552-64. [Crossref] [PubMed]
- Griepp RB, Griepp EB. Spinal cord protection in surgical and endovascular repair of thoracoabdominal aortic disease. J Thorac Cardiovasc Surg 2015;149:S86-90. [Crossref] [PubMed]
- Miranda V, Sousa J, Mansilha A. Spinal cord injury in endovascular thoracoabdominal aortic aneurysm repair: prevalence, risk factors and preventive strategies. Int Angiol 2018;37:112-26. [Crossref] [PubMed]
- Hu Z, Zhang Z, Liu H, et al. Fenestrated and Branched Stent-Grafts for the Treatment of Thoracoabdominal Aortic Aneurysms: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2022;9:901193. [Crossref] [PubMed]
- Xie W, Xue Y, Li S, et al. Left subclavian artery revascularization in thoracic endovascular aortic repair: single center's clinical experiences from 171 patients. J Cardiothorac Surg 2021;16:207. [Crossref] [PubMed]
- Marturano F, Nisi F, Giustiniano E, et al. Prevention of Spinal Cord Injury during Thoracoabdominal Aortic Aneurysms Repair: What the Anaesthesiologist Should Know. J Pers Med 2022;12:1629. [Crossref] [PubMed]
- Englund R. Review of Thoracic Endovascular Aneurysm Repair (TEVAR), Spinal Cord Ischemia (SCI), Cerebrospinal Fluid (CSF) Drainage and Blood Pressure (BP) Augmentation. Surgical Science 2017;8:73-81.
- Zhang Z, Zhou Y, Lin S, et al. Systematic review and meta-analysis of association of prophylactic cerebrospinal fluid drainage in preventing spinal cord ischemia after thoracic endovascular aortic repair. J Vasc Surg 2022;75:1478-1489.e5. [Crossref] [PubMed]
- Song S, Song SW, Kim TH, et al. Effects of preemptive cerebrospinal fluid drainage on spinal cord protection during thoracic endovascular aortic repair. J Thorac Dis 2017;9:2404-12. [Crossref] [PubMed]
- Suarez-Pierre A, Zhou X, Gonzalez JE, et al. Association of preoperative spinal drain placement with spinal cord ischemia among patients undergoing thoracic and thoracoabdominal endovascular aortic repair. J Vasc Surg 2019;70:393-403. [Crossref] [PubMed]
- Wong CS, Healy D, Canning C, et al. A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endografting. J Vasc Surg 2012;56:1438-47. [Crossref] [PubMed]
- Seike Y, Fukuda T, Yokawa K, et al. Aggressive use of prophylactic cerebrospinal fluid drainage to prevent spinal cord ischemia during thoracic endovascular aortic repair is not supportive. Eur J Cardiothorac Surg 2022;62:ezac441. [Crossref] [PubMed]
- Aucoin VJ, Bolaji B, Novak Z, et al. Trends in the use of cerebrospinal drains and outcomes related to spinal cord ischemia after thoracic endovascular aortic repair and complex endovascular aortic repair in the Vascular Quality Initiative database. J Vasc Surg 2021;74:1067-78. [Crossref] [PubMed]
- Kärkkäinen JM, Cirillo-Penn NC, Sen I, et al. Cerebrospinal fluid drainage complications during first stage and completion fenestrated-branched endovascular aortic repair. J Vasc Surg 2020;71:1109-1118.e2. [Crossref] [PubMed]
- Bisdas T, Panuccio G, Sugimoto M, et al. Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg 2015;61:1408-16. [Crossref] [PubMed]
- Juszczak MT, Murray A, Koutsoumpelis A, et al. Elective Fenestrated and Branched Endovascular Thoraco-abdominal Aortic Repair with Supracoeliac Sealing Zones and without Prophylactic Cerebrospinal Fluid Drainage: Early and Medium-term Outcomes. Eur J Vasc Endovasc Surg 2019;57:639-48. [Crossref] [PubMed]
- Kemp C, Ikeno Y, Aftab M, et al. Cerebrospinal fluid drainage in thoracic endovascular aortic repair: mandatory access but tailored placement. Ann Cardiothorac Surg 2022;11:53-5. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- IHE. Quality Appraisal of Case Series Checklist 2014. Available online: https://www.ihe.ca/publications/ihe-quality-appraisal-checklist-for-case-series-studies
- Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. Journal of Statistical Software 2012;49:1-15.
- McGrath S, Zhao X, Steele R, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res 2020;29:2520-37. [Crossref] [PubMed]
- Collaboration C. Review Manager. 5.4 ed, 2020. Available online: https://training.cochrane.org/online-learning/core-software/revman
- Acher C, Acher CW, Marks E, et al. Intraoperative neuroprotective interventions prevent spinal cord ischemia and injury in thoracic endovascular aortic repair. J Vasc Surg 2016;63:1458-65. [Crossref] [PubMed]
- Adams JD, Angle JF, Matsumoto AH, et al. Endovascular repair of the thoracic aorta in the post-FDA approval era. J Thorac Cardiovasc Surg 2009;137:117-23. [Crossref] [PubMed]
- Addas JAK, Mafeld S, Mahmood DN, et al. Minimally Invasive Segmental Artery Coil Embolization (MISACE) Prior to Endovascular Thoracoabdominal Aortic Aneurysm Repair. Cardiovasc Intervent Radiol 2022;45:1462-9. [Crossref] [PubMed]
- Angiletta D, Piffaretti G, Patruno I, et al. Preliminary results from a multicenter Italian registry on the use of a new branched device for the treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2021;74:404-13. [Crossref] [PubMed]
- Arnaoutakis DJ, Arnaoutakis GJ, Beaulieu RJ, et al. Results of adjunctive spinal drainage and/or left subclavian artery bypass in thoracic endovascular aortic repair. Ann Vasc Surg 2014;28:65-73. [Crossref] [PubMed]
- Banno H, Kawai Y, Sato T, et al. Low-density vulnerable thrombus/plaque volume on preoperative computed tomography predicts for spinal cord ischemia after endovascular repair for thoracic aortic aneurysm. J Vasc Surg 2021;73:1557-1565.e1. [Crossref] [PubMed]
- Bobadilla JL, Wynn M, Tefera G, et al. Low incidence of paraplegia after thoracic endovascular aneurysm repair with proactive spinal cord protective protocols. J Vasc Surg 2013;57:1537-42. [Crossref] [PubMed]
- Chaudhary O, Sharkey A, Schermerhorn M, et al. Protocolized Based Management of Cerebrospinal Fluid Drains in Thoracic Endovascular Aortic Aneurysm Repair Procedures. Ann Vasc Surg 2021;72:409-18. [Crossref] [PubMed]
- Cheung AT, Pochettino A, McGarvey ML, et al. Strategies to manage paraplegia risk after endovascular stent repair of descending thoracic aortic aneurysms. Ann Thorac Surg 2005;80:1280-8; discussion 1288-9. [Crossref] [PubMed]
- Chuter TA, Rapp JH, Hiramoto JS, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2008;47:6-16. [Crossref] [PubMed]
- D'Oria M, Chiarandini S, Pipitone M, et al. Coverage of visible intercostal and lumbar segmental arteries can predict the volume of cerebrospinal fluid drainage in elective endovascular repair of descending thoracic and thoracoabdominal aortic disease: a pilot study. Eur J Cardiothorac Surg 2019;55:646-52. [Crossref] [PubMed]
- D'Souza S, Duncan A, Aguila F, et al. TEVAR for non-aneurysmal thoracic aortic pathology. Catheter Cardiovasc Interv 2009;74:783-6. [Crossref] [PubMed]
- Desart K, Scali ST, Feezor RJ, et al. Fate of patients with spinal cord ischemia complicating thoracic endovascular aortic repair. J Vasc Surg 2013;58:635-42.e2. [Crossref] [PubMed]
- Fossaceca R, Guzzardi G, Cerini P, et al. Endovascular treatment of thoracic aortic aneurysm: a single-center experience. Ann Vasc Surg 2013;27:1020-8. [Crossref] [PubMed]
- Hiraoka T, Komiya T, Tsuneyoshi H, et al. Risk factors for spinal cord ischaemia after thoracic endovascular aortic repair. Interact Cardiovasc Thorac Surg 2018;27:54-9. [Crossref] [PubMed]
- Hnath JC, Mehta M, Taggert JB, et al. Strategies to improve spinal cord ischemia in endovascular thoracic aortic repair: Outcomes of a prospective cerebrospinal fluid drainage protocol. J Vasc Surg 2008;48:836-40. [Crossref] [PubMed]
- Iafrancesco M, Ranasinghe AM, Claridge MW, et al. Current results of endovascular repair of thoraco-abdominal aneurysms. Eur J Cardiothorac Surg 2014;46:981-4. [Crossref] [PubMed]
- Iyer VS, Mackenzie KS, Tse LW, et al. Early outcomes after elective and emergent endovascular repair of the thoracic aorta. J Vasc Surg 2006;43:677-83. [Crossref] [PubMed]
- Kato M, Motoki M, Isaji T, et al. Spinal cord injury after endovascular treatment for thoracoabdominal aneurysm or dissection. Eur J Cardiothorac Surg 2015;48:571-7. [Crossref] [PubMed]
- Khoynezhad A, Azizzadeh A, Donayre CE, et al. Results of a multicenter, prospective trial of thoracic endovascular aortic repair for blunt thoracic aortic injury (RESCUE trial). J Vasc Surg 2013;57:899-905.e1. [Crossref] [PubMed]
- Kitpanit N, Ellozy SH, Connolly PH, et al. Risk factors for spinal cord injury and complications of cerebrospinal fluid drainage in patients undergoing fenestrated and branched endovascular aneurysm repair. J Vasc Surg 2021;73:399-409.e1. [Crossref] [PubMed]
- Kotelis D, Bianchini C, Kovacs B, et al. Early experience with automatic pressure-controlled cerebrospinal fluid drainage during thoracic endovascular aortic repair. J Endovasc Ther 2015;22:368-72. [Crossref] [PubMed]
- Maier S, Shcherbakova M, Beyersdorf F, et al. Benefits and Risks of Prophylactic Cerebrospinal Fluid Catheter and Evoked Potential Monitoring in Symptomatic Spinal Cord Ischemia Low-Risk Thoracic Endovascular Aortic Repair. Thorac Cardiovasc Surg 2019;67:379-84. [Crossref] [PubMed]
- Maurel B, Delclaux N, Sobocinski J, et al. The impact of early pelvic and lower limb reperfusion and attentive peri-operative management on the incidence of spinal cord ischemia during thoracoabdominal aortic aneurysm endovascular repair. Eur J Vasc Endovasc Surg 2015;49:248-54. [Crossref] [PubMed]
- Mazzeffi M, Abuelkasem E, Drucker CB, et al. Contemporary Single-Center Experience With Prophylactic Cerebrospinal Fluid Drainage for Thoracic Endovascular Aortic Repair in Patients at High Risk for Ischemic Spinal Cord Injury. J Cardiothorac Vasc Anesth 2018;32:883-9. [Crossref] [PubMed]
- Nathan DP, Shalhub S, Tang GL, et al. Outcomes after stent graft therapy for dissection-related aneurysmal degeneration in the descending thoracic aorta. J Vasc Surg 2015;61:1200-6. [Crossref] [PubMed]
- Pasqualucci A, Al-Sibaie A, Vaidyan KPT, et al. Epidural Corticosteroids, Lumbar Spinal Drainage, and Selective Hemodynamic Control for the Prevention of Spinal Cord Ischemia in Thoracoabdominal Endovascular Aortic Repair: A New Clinical Protocol. Adv Ther 2020;37:272-87. [Crossref] [PubMed]
- Preventza O, Wheatley GH 3rd, Williams J, et al. Identifying paraplegia risk associated with thoracic endografting. Asian Cardiovasc Thorac Ann 2009;17:568-72. [Crossref] [PubMed]
- Rizk MAEMAES, Ismail MIM, Gohar KS. Stroke, spinal cord ischemia and upper limb ischemia in patients undergoing TEVAR with coverage of the left subclavian artery: a case series study. Egypt J Radiol Nucl Med 2021;52:276.
- Schurink GW, Nijenhuis RJ, Backes WH, et al. Assessment of spinal cord circulation and function in endovascular treatment of thoracic aortic aneurysms. Ann Thorac Surg 2007;83:S877-81; discussion S890-2. [Crossref] [PubMed]
- Sugiyama Y, Fuseya S, Aiba K, et al. Preoperative and postoperative complications of cerebrospinal fluid drainage in descending thoracic and thoraco-abdominal aortic aneurysm surgery: a single-center retrospective study. J Anesth 2022;36:476-83. [Crossref] [PubMed]
- Sulzinski MC, Rossi MJ, Alfawaz AA, et al. Optimization of factors for the prevention of spinal cord ischemia in thoracic endovascular aortic repair. Vascular 2022;30:199-205. [Crossref] [PubMed]
- Verma M, Ojha V, Deshpande AA, et al. Association between aortic coverage and spinal cord ischemia after endovascular repair of type B aortic dissection. Indian J Thorac Cardiovasc Surg 2022;38:375-81. [Crossref] [PubMed]
- Verzini F, Ferrer C, Parlani G, et al. Mid-Term Outcomes of Complex Endografting for Chronic Post-Dissection Thoracoabdominal Aortic Aneurysms. Cardiovasc Intervent Radiol 2020;43:1440-8. [Crossref] [PubMed]
- Yang GK, Misskey J, Arsenault K, et al. Outcomes of a Spinal Drain and Intraoperative Neurophysiologic Monitoring Protocol in Thoracic Endovascular Aortic Repair. Ann Vasc Surg 2019;61:124-33. [Crossref] [PubMed]
- Zipfel B, Buz S, Redlin M, et al. Spinal cord ischemia after thoracic stent-grafting: causes apart from intercostal artery coverage. Ann Thorac Surg 2013;96:31-8. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9. [Crossref] [PubMed]
- Cinà CS, Abouzahr L, Arena GO, et al. Cerebrospinal fluid drainage to prevent paraplegia during thoracic and thoracoabdominal aortic aneurysm surgery: a systematic review and meta-analysis. J Vasc Surg 2004;40:36-44. [Crossref] [PubMed]
- Wynn MM, Sebranek J, Marks E, et al. Complications of spinal fluid drainage in thoracic and thoracoabdominal aortic aneurysm surgery in 724 patients treated from 1987 to 2013. J Cardiothorac Vasc Anesth 2015;29:342-50. [Crossref] [PubMed]
- Estrera AL, Sheinbaum R, Miller CC, et al. Cerebrospinal fluid drainage during thoracic aortic repair: safety and current management. Ann Thorac Surg 2009;88:9-15; discussion 15. [Crossref] [PubMed]
- Dardik A, Perler BA, Roseborough GS, et al. Subdural hematoma after thoracoabdominal aortic aneurysm repair: an underreported complication of spinal fluid drainage? J Vasc Surg 2002;36:47-50. [Crossref] [PubMed]
- Rong LQ, Kamel MK, Rahouma M, et al. Cerebrospinal-fluid drain-related complications in patients undergoing open and endovascular repairs of thoracic and thoraco-abdominal aortic pathologies: a systematic review and meta-analysis. Br J Anaesth 2018;120:904-13. [Crossref] [PubMed]
- Spanos K, Kölbel T, Kubitz JC, et al. Risk of spinal cord ischemia after fenestrated or branched endovascular repair of complex aortic aneurysms. J Vasc Surg 2019;69:357-66. [Crossref] [PubMed]
- Katsargyris A, Oikonomou K, Kouvelos G, et al. Spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms with fenestrated and branched stent grafts. J Vasc Surg 2015;62:1450-6. [Crossref] [PubMed]







