Valve-sparing operations after Ross procedure: a single-center experience
Introduction
The Ross procedure replaces the dysfunctional aortic valve with the pulmonary autograft (1). It is considered a living valve substitute, and therefore has the ability to grow with the patient. As such, it had gained wide popularity in the pediatric population since its introduction in 1967. Thus, in pediatric patients in whom the aortic valve was deemed not repairable, it was the ideal valve substitute with good long-term results. In the adult population, however, it hadn’t gained much traction until more recently, when long-term results consistently demonstrated that it is the only aortic valve replacement strategy which is able to restore normal life-expectancy. Other advantages are lower transvalvular gradients with resultant optimized hemodynamics, a decreased incidence of endocarditis, and the obviation of the need for long-term anticoagulation. Thus, not only does it restore life-expectancy, but it also restores a nearly normal quality-of-life. Nonetheless, a weakness of this technique is the neo-aortic root enlargement over time, which can lead to reoperation for the related neo-aortic valve dysfunction and/or the aforementioned aortic root enlargement itself. This is particularly prevalent in the free root technique, where a pulmonary autograft without external support, which is designed for a low-pressure system (the pulmonary circulation), is perpetually exposed to a high-pressure system (the systemic circulation). In these circumstances, a redo surgery is sometimes required later in life, to address the respective pathology. Our center’s philosophy is valve-preservation surgery, whenever appropriate. Hence, we are sharing our experience with valve-sparing reoperations after Ross procedures.
Methods
Study design
We searched our prospectively collected institutional database for aortic valve repair, for all patients who have undergone valve-sparing surgeries (e.g., remodeling or reimplantation technique, and/or aortic valve-repair) after a previous Ross operation. All adult patients (>18 years), who have undergone valve-sparing surgery after Ross between July 2001 and July 2022 at a single-institution (Cliniques Universitaires Saint-Luc, Brussels, Belgium), were recruited for this study. The indication for valve-sparing root replacement (VSSR) and/or aortic valve repair was pulmonary autograft aneurysm and/or severe aortic regurgitation (AR). Clinical follow-up data was collected via telephone or was provided by the referring cardiologist, or was gathered from hospital records, whenever available. Clinical data was reported according to the 2008 Society of Thoracic Surgeons/American Association for Thoracic Surgery/European Association for Cardio-Thoracic Surgery guidelines (2). Early mortality was defined as any death occurring during initial hospital stay, or during the first 60 days following the operation; any other mortality events were considered late deaths. This study was approved by the ethics review board of our academic institution [Université Catholique de Louvain (ID Brussels: 2013/03JUI/36)].
Surgical technique
The majority of patients who required neo-aortic root replacement after Ross procedure, underwent reimplantation (David procedure) of the neo-aortic valve. This was done after redo-sternotomy, with either central or femoral cannulation, and after evaluation with a preoperative computed tomography (CT)-scan of the chest. The heart was arrested via antegrade normothermic blood cardioplegia, and after the aorta was cross-clamped and opened, cardioplegia was administered via direct coronary cannulation every 20 min. A left ventricular vent is placed through the right upper pulmonary vein. The aortic root was then dissected circumferentially to the level of the virtual basal ring (VBR) externally. The non-coronary sinus was excised first, leaving a 3–4 mm remnant of neo-aortic tissue. The right coronary button was then excised, followed by the left coronary button. In our early experience of the Ross procedure, a pericardial strip or prosthetic material was used at the level of the surgical annulus, which can cause severe calcifications later. We now avoid using any material for the proximal Ross suture line. However, whenever calcifications from previous prosthetic materials are encountered, they are aggressively debrided before seating the Valsalva-graft.
Sub-valvular sutures are then placed at the level of the VBR, except for the area of the membranous septum, where placement follows aortic cusp insertion. We use plegeted 2.0 Ethibond sutures. One at each commissure and three for each sinus for a total of 12, in tricuspid aortic valves or neo-aortic valves (pulmonary autografts). The sutures are then placed through the bottom of the Valsalva portion of the Valsalva- (Gelweave Valsalva-graft, Terumo, Leuven, Belgium) or Cardioroot-graft (Cardioroot, Getinge, Sweden). The graft is sized according to the height of the non/left commissure, as previously described by our group (3,4). The graft is brought down to the aortic annulus (VBR), and all sutures are tied. The three commissures of the neo-aortic valve are then resuspended and secured at the neo-sinotubular junction (STJ) with 4.0 prolene sutures. The sutures are tied at the STJ first, and then run from commissure to commissure to secure the neo-aortic remnant to the graft, as described before for the native aortic root (4). The valve is then assessed, and a prolapse is treated with a central cusp plication as needed, utilizing a 5.0 or 6.0 prolene suture, depending on the quality of cusp tissues (5). The coronary arteries are reimplanted into the graft with a 5.0 prolene suture. Occasionally, we will reinforce the coronary artery suture line with a strip of autologous pericardium. In patients who underwent the remodeling technique (Yacoub procedure), the root was dissected in a similar fashion, without the deep root dissection required for the reimplantation technique, however. A straight tube graft is then sized similar to the reimplantation technique, and one to three tongues are fashioned to sew to the aortic remnant with a 4.0 prolene suture, depending on how many sinuses require excision. The coronary arteries are then reimplanted in a similar fashion as in the reimplantation technique, but this is only required when the coronary sinuses are resected and replaced.
In patients who did not require a root replacement, we perform central cusp plications to treat a prolapse. The technique has been described in detail in the past (5). Briefly, when performing central cusp plication, the other two cusps are used as reference cusps. The plication is made at the middle of the leaflet going from aorta to ventricle, and then ventricle to aorta. This is then tied, and sometimes reinforced with an additional figure-of-eight suture. When there is annular enlargement at the level of the VBR, then a commissural Cabrol annuloplasty or and external annuloplasty can be added. The distal aortic anastomosis is then performed and the cross-clamped removed, the heart is re-perfused, and bypass is slowly weaned off. The valve is then assessed via trans-esophageal echocardiography.
Statistical analysis
Categorical data are presented as counts with proportions. Continuous data are presented as means (standard deviation; range) when normally distributed, or medians [interquartile range (IQR)] when not normally distributed. Univariable logistic regression analysis was performed to study potential variables affecting early mortality. Survival, freedom-from-valve-reintervention and freedom-from-AR grade ≥3 were analyzed with the Kaplan-Meier estimator, and for the analysis of association of variables (i.e., predictors) with outcome (P value of <0.1, or clinically relevant), the Cox regression model was used. All tests were performed two-sided, and a P value of <0.05 was considered statistically significant. For the hazard of late death and reintervention, the following variables were considered: age, gender, body mass index (BMI), preoperative New York Heart Association (NYHA) class, preoperative left ventricle ejection fraction, left ventricular end-diastolic diameter (LVEDD), chronic renal failure, pulmonary hypertension, chronic obstructive pulmonary disease, peripheral artery disease, diabetes, indication for surgery (i.e., aneurysm, aneurysm + AR or isolated AR), connective tissue disorder, concomitant procedures. Since there were no variables significantly associated with outcome in the univariable analysis, and also due to the small number of events, we did not perform a multivariable analysis. To account for informative censoring, survival and freedom-from-reintervention were presented with the cumulative incidence function, from a competing risk analysis (using the R “cmprsk” package). For the statistical analysis R (version 4.2.0, available at: https://www.r-project.org/) and GraphPad Prism version 9.3.1 for Windows, GraphPad Software, San Diego, CA, USA, https://www.graphpad.com/, were used.
Results
Demographics
During the study period, a total of 63 patients with a previous Ross procedure underwent a VSRR operation with the reimplantation (n=39) or remodeling (n=3) technique and/or autograft neo-aortic valve repair (n=21). Indication for operation was aortic aneurysm without AR in 17 (27%) patients, aortic aneurysm with AR in 27 (43%) patients, and isolated AR in 19 (30%). The median follow-up time was 7.82 years (IQR, 4.4–14.15 years). Patient characteristics are summarized in Table 1. All operative characteristics are shown in Table 2.
Table 1
| Patient characteristics | Value |
|---|---|
| Total, n | 63 |
| Age (years), mean | 41.1 |
| BMI (kg/m2), mean | 26.3 |
| Male (%) | 86.0 |
| Indication for operation (%) | |
| AR | 30.2 |
| Ascending aneurysm | 27.0 |
| AR + aneurysm | 42.8 |
| Endocarditis | 1.6 |
| NYHA (%) | |
| I | 49.2 |
| II | 34.9 |
| III | 15.9 |
| IV | 0.0 |
| Sinus rhythm (%) | 98.0 |
| Atrial fibrillation (%) | 0.0 |
| Pacemaker (%) | 2.0 |
| Preop echo (LVEF) (%) | |
| Good (>50%) | 76.2 |
| Moderate (31–50%) | 22.2 |
| Poor (21–30%) | 1.6 |
| Very poor (<21%) | 0.0 |
| COPD (%) | 1.6 |
| Connective tissue disease (%) | 0.0 |
| Peripheral arterial disease (%) | 0.0 |
| Diabetes mellitus (%) | 0.0 |
| Pulmonary hypertension (PAPmean) (%) | |
| PAP <30 mmHg | 84.1 |
| PAP 31–55 mmHg | 12.7 |
| PAP >55 mmHg | 3.1 |
BMI, body mass index; AR, aortic regurgitation; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; COPD, chronic obstructive pulmonary disease; PAP, pulmonary artery pressure.
Table 2
| Operative characteristics | Value |
|---|---|
| Type of repair, n (%) | |
| Isolated valve repair | 11 (17.46) |
| Tubular aorta replacement ± valve repair | 9 (14.29) |
| Partial root replacement (1–2 sinus) ± valve repair | 1 (1.59) |
| Valve sparing root replacement ± valve repair | 42 (66.67) |
| Type of VSRR (n=42), n (%) | |
| David | 39 (92.86) |
| Yacoub | 3 (7.14) |
| Sinus-type graft (Valsalva or Cardioroot), n | 36 |
| Concomitant procedures, n (%) | |
| CABG | 3 (4.76) |
| (Hemi)arch | 0 (0.00) |
| Mitral, n (%) | 3 (4.76) |
| Pulmonary valve replacement, n (%) | 22 (34.90) |
| CPB time (min) | 155 |
| Cross clamp time (min) | 119 |
| 2nd cross clamp (n=8) | |
| AR > grade 2 | 6 |
| MV regurgitation | 1 |
| Bleeding | 1 |
VSRR, valve-sparing root replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; AR, aortic regurgitation; MV, mitral valve.
Initial Ross technique
Of the study cohort:
- 48 patients underwent a free-root technique (76%);
- 4 patients underwent sub-coronary autograft implantation (6%);
- 7 patients underwent native aorta inclusion (11%);
- 4 patients underwent prosthetic-graft inclusion (6%).
Interestingly, the majority of patients underwent reoperation after the free-root technique. The four patients who required reoperation after graft inclusion did not have dilation of the neo-root but required cusp repairs for cusp prolapse. Six out of seven patients in the native aorta inclusion technique required cusp repairs only; one patient also had a root dilation, and one had an ascending dilation. All four patients in the sub-coronary technique had neo-root dilation. In our patient experience thus far, only the graft inclusion technique provided 100% freedom from autograft dilation.
Outcomes
Early outcomes
Only one patient died in the early postoperative period (1.59%), nine days after the procedure due to cardiac arrest. There were no other early complications and no variables were associated with early mortality.
Late survival
A total of three patients died during follow-up, one from valve-related causes, one died from cancer, and one patient committed suicide in the setting of alcoholism and a history of psychiatric disorders. Cumulative survival after redo surgery following Ross was 98.4% [95% confidence interval (CI): 89.3–99.8%] at 1 year, 96.3% (95% CI: 88.2–98.3%) at 5 years, and 92.42% (95% CI: 87.1–98.0%) at 10 years. Figure 1 shows the overall survival. Univariable cox regression model identified no variables as predictors for late mortality.
Late reoperations
Freedom-from-reoperation on the aortic valve at 1 year was 98.4% (95% CI: 97.0–99.8%), at 5 years was 96.7% (95% CI: 87.6–99.0%), and 79.7% (95% CI: 71.1–88.3%) at 10 years (Figure 2). A univariable cox regression analysis identified no variables as predictors for late reoperation. Figure 2 shows freedom-from-reintervention on the aortic valve and Figure 3 shows the competing risk of the cumulative incidence of death and mortality. A total of 10 patients underwent reoperation on the aortic valve. Reason for reoperations were moderate to severe, or severe symptomatic AR (n=6), endocarditis (n=3), and unknown in one patient due to lack of documentation. Of these patients, 8 underwent a prosthetic aortic valve replacement and two underwent a successful re-repair of the valve.
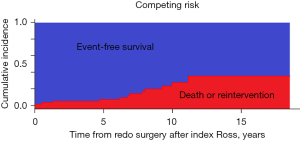
Other valve-related outcomes
Figure 4 shows freedom-from-AV regurgitation of grade 3 or more during follow-up, verified by echocardiography. Moreover, three patients developed endocarditis during follow-up and were successfully reoperated. Another three patients had a thromboembolic event (1 TIA and 2 strokes). One patient with a poor left ventricular ejection fraction before reoperation was hospitalized for heart failure and placed on the heart transplant wait list.
Discussion
The Ross procedure is commonly employed at our institution, especially in young patients, who aren’t aortic valve repair candidates (e.g., severe aortic stenosis), with results that mirror the survival of the general population (6). Depending on the underlying pathology and dimensions of the functional aortic annulus, we individualize the surgical strategy. There are several techniques for implantation of the pulmonary autograft onto the surgical annulus of the aortic valve. They range from a (I) sub-coronary technique, similar to early stentless valves; to the (II) free root technique; the (III) native aortic root inclusion; and the (IV) prosthetic graft inclusion technique (7). Although all strategies were employed at our center at earlier time periods, we now commonly focus on the latter three techniques (Figure 5).
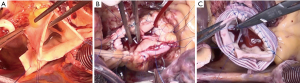
The native aorta, or prosthetic graft inclusion technique, lends support to the functional annulus of the pulmonary autograft, thereby in theory, preventing neo-aortic root dilation, which will also lead to stable neo-aortic valve function over time. In this study, however, we now find that not all inclusion techniques are equally protective from neo-aortic root dilation. In the sub-coronary inclusion technique, all 4 patients suffered neo-aortic root dilation, and one of the 7 patients with the native aorta inclusion technique. Only the prosthetic graft inclusion technique was 100% protective from neo-aortic root dilation. This highlights the notion that the native aorta can grow with the pulmonary autograft and lead to autograft dysfunction over time, despite the respective inclusion technique.
This study, however, demonstrates that good long-term survival can be achieved despite neo-aortic root dilation or neo-aortic valve dysfunction after the index Ross operation, with 10-year survival of 92% and freedom-from-reoperation on the neo-aortic valve at 10-year of close to 80%, despite the required repair of the neo-aortic valve or neo-root replacement. In a recent study from Texas, Shih et al. demonstrated the feasibility of reintervention after the Ross procedure with restoration of normal life expectancy, despite the necessary reintervention later on (8,9). The authors, however, performed more valve replacements than in our cohort of patients (40 out of 66 patients), where we generally aim at valve-preservation first. Herein, we have demonstrated that reintervention is safe, neo-aortic valve preservation is feasible, and can achieve excellent long-term survival and satisfactory freedom-from-reoperation on the neo-aortic valve at 10 years. Multiple recent studies from Europe and North America have now consistently demonstrated that outcomes after the Ross procedure are superior to outcomes after prosthetic aortic valve replacement over the course of 20 years (10-12). Moreover, at the recent Annual Meeting of the American Association for Thoracic Surgery (AATS; May 2023), Sir Magdi Yacoub presented his very long data on the Ross procedure, and provided excellent survival and freedom from reintervention into the third decade in young adults (13).
However, we should not discount the fact that Ross procedures are complex operations with potential for serious harm to adjacent structures, such as the coronary arteries, and should thus never be taken lightly. Because the native stent of the pulmonary autograft is less sturdy than the native aortic valve stent, as it is designed for a low-pressure system, it is also more prone to distortion of its’ valve geometry during implantation. These potential pitfalls need to be recognized and taken into consideration, when performing these complex procedures. Ideally, these operations should be performed at high-volume centers of excellence, for not only the Ross operation, but also for aortic valve repair and valve-sparing surgeries, as these skills can come in handy during the index operation, but also during redo surgeries.
Study limitations
This is a single-center retrospective analysis with all its’ inherent shortcomings. Although this study reflects upon our experience of reinterventions after Ross operations, it does not summarize our entire Ross experience. We were not able to correctly delineate the number of patients who underwent their index Ross operation at our institution or elsewhere, as this was not reflected in our aortic valve repair prospective database, which was used for this analysis. Although this is the largest cohort of patients who have undergone valve-sparing operations after the Ross procedure to date, it is still a limited experience, and larger experiences are needed to confirm our findings. Due to the relatively low power of the study, certain risk factors, such as root dilation and/or AR, were not found to be predictors for reoperation, but this will likely change with a greater patient population in our opinion.
Conclusions
Autograft dilatation and/or aortic valve dysfunction can occur after the Ross operation, but is amenable to repair and valve-preservation, with excellent long-term results thereafter. Only the prosthetic-graft inclusion technique has demonstrated a 100% freedom-from-autograft dilation at 10-year and should therefore be considered for all patients with known risk factors. The data support our aggressive approach for valve-preservation after the Ross procedure.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967;2:956-8. [Crossref] [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;85:1490-5. [Crossref] [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. A new simple and objective method for graft sizing in valve-sparing root replacement using the reimplantation technique. Ann Thorac Surg 2011;92:749-51. [Crossref] [PubMed]
- Jahanyar J, de Kerchove L, El Khoury G. Bicuspid aortic valve repair: the 180°-Reimplantation technique. Ann Cardiothorac Surg 2022;11:473-81. [Crossref] [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. Cusp prolapse repair in trileaflet aortic valves: free margin plication and free margin resuspension techniques. Ann Thorac Surg 2009;88:455-61; discussion 461. [Crossref] [PubMed]
- Mastrobuoni S, de Kerchove L, Solari S, et al. The Ross procedure in young adults: over 20 years of experience in our Institution. Eur J Cardiothorac Surg 2016;49:507-12; discussion 512-3. [Crossref] [PubMed]
- Jahanyar J, Muñoz DE, Tamer S, et al. The Ross inclusion Dacron graft. Ann Cardiothorac Surg 2021;10:549-51. [Crossref] [PubMed]
- Shih E, Brinkman WT, Harrington KB, et al. Outcomes of redo operations after the Ross procedure. J Thorac Cardiovasc Surg 2023;165:1803-1812.e2. [Crossref] [PubMed]
- Jahanyar J, Mastrobuoni S, de Kerchove L, et al. Commentary: Keeping Ross on its original trajectory. J Thorac Cardiovasc Surg 2023;165:1813-4. [Crossref] [PubMed]
- El-Hamamsy I, Toyoda N, Itagaki S, et al. Propensity-Matched Comparison of the Ross Procedure and Prosthetic Aortic Valve Replacement in Adults. J Am Coll Cardiol 2022;79:805-15. [Crossref] [PubMed]
- Gofus J, Fila P, Drabkova S, et al. Ross procedure provides survival benefit over mechanical valve in adults: a propensity-matched nationwide analysis. Eur J Cardiothorac Surg 2022;61:1357-65. [Crossref] [PubMed]
- Mazine A, David TE, Stoklosa K, et al. Improved Outcomes Following the Ross Procedure Compared With Bioprosthetic Aortic Valve Replacement. J Am Coll Cardiol 2022;79:993-1005. [Crossref] [PubMed]
- Melina G, Notenboom M, Veen K, et al. Survival and Reinterventions After the Ross Procedure in Adults: A 28 Year Follow-Up Study. 2023. Available online: https://www.aats.org/resources/survival-and-reinterventions-after-the-ross-procedure-in-adults-a-28-year-follow-up-study









