Transcatheter options for atrial fibrillation treatment: an overview of the ablative techniques currently available and future perspectives
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in the adult population and catheter ablation (CA) for AF has emerged as an important rhythm-control strategy and is the most common cardiac ablation procedure performed worldwide (1). In the late 90s, the identification by Haïssaguerre et al. of pulmonary veins (PVs) electrical activity as the main trigger for AF occurrence (2) led the electrophysiologist community to abandon the attempts to replicate surgical lines developed by Cox and colleagues (3). It has become increasingly evident that the presence of excitable tissues within the PVs and at the left atrium (LA)-PV junction plays a central role in AF occurrence. Following this, the main ablative strategy developed was to interrupt the electrical connections between PVs and the remaining LA. This technique evolved over time from a segmental approach, targeting the earliest site of activation at PV musculature close to the ostium (4), to an ablation strategy aimed at PVs encircling and guided by an electro-anatomic mapping system (5). Pulmonary vein isolation (PVI) has demonstrated a success rate between 60% to 90% at 12-month follow-up in patients with paroxysmal AF, with a wide variation depending on ECG monitoring during the follow-up, but consistently superior to antiarrhythmic drugs (AAD) in multiple randomized controlled trials (6-8). A durable PVI has a pivotal role in the maintenance of sinus rhythm and in the last few years, many efforts by the scientific community have been made to reduce the PV reconnections. The left atrial wall is a thin structure with heterogeneous wall thickness, ranging from 1 to 5 mm, with an important inter- and intra-patient variability. Non-transmural ablation is considered a major determinant of post-ablation AF recurrence; El Haddad et al. showed that PV reconnection could be due to insufficient lesion depth (9). Nevertheless, PVs are not the only players in the complex phenomenon of AF genesis and maintenance. Although PVI remains the cornerstone of AF ablation, in persistent and long-standing persistent AF, post-ablation long-term outcomes have been significantly less favorable than in paroxysmal AF, reporting a success rate between 40% and 70% (10) and often requiring repeated procedures. Extra-PVs triggers, interstitial fibrous tissue deposition and/or autonomic innervation are probably involved, even if the magnitude of their implication in AF occurrence is still not completely understood and, probably, varies from one patient to another. Ablation strategies combining PVI with additional extra-PV substrate ablation, such as either linear ablation, complex fractionated atrial electrogram ablation, or MRI-guided fibrosis ablation, have been tested to further increase the efficacy of CA but showed mixed results (11,12). Accordingly, a PVI-only approach using radiofrequency (RF) or cryoballoon, with post-ablation confirmation of both entrance and exit block, is the most widespread and used technique. On the other hand, the possibility of injuring some sensitive extracardiac structures represents a major issue up to now, especially near regions where the LA thickness is lower. The introduction of non-thermal energy ablation, such as electroporation, could overcome this limitation, thus representing a game-changer. This review seeks to provide an update report of the current practices and approaches in the field of transcatheter AF ablation, and to describe the latest advances in technology that aim to improve procedural safety, efficacy and to reduce procedural requirements in terms of duration and fluoroscopy exposure.
Current indications for catheter ablation of AF
Evidence regarding the efficacy of CA has been growing in the last decade. AF ablation has been shown to be safe and more effective than AAD for the maintenance of sinus rhythm and for the reduction of symptoms (7,8,13-22). So far, no randomized clinical trial was able to demonstrate mortality reduction in the general AF population. Therefore, indications for CA are mostly based on the presence of symptoms. The current guidelines recommend CA as class I indication in patients in whom AADs have failed or are not tolerated (1), whereas CA can be proposed as first-line rhythm control therapy (IIa class indication) to improve symptoms in selected patients with symptomatic AF episodes. These current indications are soon to be changed, taking into consideration the results from the EAST trial (23), which showed benefit on hard endpoints even in asymptomatic patients undergoing early rhythm control. Moreover, the EARLY-AF trial (7) showed a higher rate of progression into persistent AF in the AAD arm compared to first-line cryoablation and should push the next guidelines to put first-line ablation as a class I indication at least in patients with high AF-burden. The evidence regarding the benefits of CA in patients with heart failure are rising. Ablation have shown to improve left ventricular ejection fraction (LVEF), quality of life and functional capacity in a recent meta-analysis (24). The CASTLE-AF study (19) showed for the first time a benefit in post-ablation survival in a population of patients with AF, New York Heart Association (NYHA) class II–IV and LVEF <35%. Compared to medical therapy, ablation was associated with a significant reduction of the composite endpoint of death or hospitalization for heart failure decompensation. A recent sub-analysis of the CABANA trial (25) selecting patients with heart failure shows that catheter ablation significantly improved survival, freedom from AF recurrences and quality of life compared with AAD. Despite this growing evidence, further data is still needed for optimizing the selection of ablation candidates as well as for selecting the most appropriate timing for catheter ablation in patients with left ventricular systolic dysfunction.
Pulmonary veins isolation: techniques, technologies and energy sources
PVI can be achieved through a point-by-point or a one-shot technique (26,27). For the former, the current standard of care is to perform a RF ablation with PVI only approach, and involves the use of three-dimensional (3D) anatomical mapping and a wide-area circumferential ablation line that is deployed with a linear catheter at the PV-antrum junction, aiming to get a continuity between points and with verification of bidirectional conduction block (28). On the other hand, the goal of the one-shot techniques is to deliver few applications (ideally only one) per vein in order to achieve a complete PV isolation. Either way, one of the major determinants in the lesion quality remains the type of energy used. Briefly, we can divide energy sources into thermal energy (RF, cryoablation, laser) and non-thermal energy (electroporation, ethanol). All the energies available can be delivered with a one-shot technique, whereas only radiofrequency can be used to perform a point-by-point technique.
Radiofrequency ablation
RF was the first energy adopted for AF ablation and is still the most used worldwide. It is generated at a frequency of 500–1,000 KHz and transmitted between the catheter tip and a skin patch. Even if RF single-shot catheters are currently available (29), RF is mostly delivered with a point-by-point approach. However, this technique is demanding and associated with a long learning curve. Over time, there was significant improvement of RF catheter technologies in order to create a homogeneous ablation lesion set without reducing the safety. The introduction of irrigated-tip catheters was pivotal in this direction (16). Usually, RF ablation with irrigated-tip catheters consists of the delivery of moderate power (20 to 40 W) for 20 to 40 seconds. The amount of energy delivered, the contact and stability are the main determinants of the formation of a transmural and durable lesion. The introduction of ablation catheter with contact force (CF)-sensing capabilities allowed a real-time estimation of both the catheter contact with the atrial tissue and catheter stability. Compared to non-CF sensing catheters, the use of real-time CF-sensing catheters for persistent AF ablation with the aim of a CF of 10–20 g during ablation is associated with shorter procedure time, shorter fluoroscopy time and a reduction in arrhythmia recurrences (30). The introduction of non-fluoroscopic 3D navigation systems allowed improvement of the continuity of the ablation line and localization of the delivered energy (5). The antral approach guided by non-fluoroscopic 3D navigation systems reduced the rates of pulmonary vein stenosis associated with AF ablation. Several studies demonstrated that the introduction of novel markers of ablation lesion quality, such as ablation index (AI) or lesion size index, permitted accurate predictions of the depth and size of the ablation lesions evaluating a composite of power, force, stability and duration (31). Previous analysis also identified that minimum AI values of 540 and 380 were predictive of freedom from acute reconnection in the anterior/roof and the posterior/inferior segments of PVs antrum, respectively. In the CLOSE protocol, it firstly described a new ablation protocol aimed for an interlesion distance of ≤6 mm, an AI ≥400 at the posterior/inferior wall, and ≥550 at the anterior/superior wall, demonstrating a high-rate of recurrence-free survival at long-term follow-up in a cohort of patients with paroxysmal AF (28). In the CLOSE to CURE study (32), 105 patients underwent RF ablation for paroxysmal AF according to the CLOSE protocol; median atrial arrhythmia burden decreased from 2.68% at baseline to 0% during the first year and remained at 0% during the second year; moreover, single-procedure recurrence-free survival was 87% at one year and 78% at two years, and quality of life improved significantly across all scores. In the recent large non-randomized Surpoint trial (33), Di Biase et al. reported the excellent safety and effectiveness outcomes of a standardized AI-guided ablation protocol for paroxysmal AF ablation.
Besides, interest for the high-power short-duration (HPSD) RF ablation is currently growing. This recently explored approach involves the delivery of high-power (ranging from 50 to 90 W) for four to ten seconds, minimizing conductive heating and increasing resistive heating. The idea behind this innovation is that HPSD ablation may minimize the impact of catheter stability as the energy emission is over a shorter period. This approach is feasible and minimizes the RF requirement, whilst maintaining high recurrence-free survival rate (34). Safety seems to improve because of the reduction of lesion depth due to the minimization of conductive heating, but data from randomized trials is still lacking.
Despite these technological improvements some issues regarding RF as an energy source persist. RF is not a cardio-selective energy and as a consequence, the possibility of damage of extracardiac structures remains a major concern. Even if uncommon, atrio-esophageal fistula remains a dreadful complication due to its dramatic consequences. To modulate RF delivery according to intra-oesophageal temperature monitoring seems not to affect the probability of developing oesophageal lesions (35), but the international consensus on AF ablation still recommends oesophageal temperature probe as Class IIa indication to monitor oesophageal temperature and to titrate RF delivery (1). Finally, steam pop due to overheating of myocardial tissue remains a safety concern for thermal ablation.
Cryoballoon ablation
Unlike RF ablation, cryoablation is usually performed with a single-shot approach. The contemporary transvenous cryoballoon catheter design consists of a deflectable catheter with a cryoballoon that is deployed using an “over-the-wire” technique, utilizing a central lumen that permits the positioning of a guidewire into the PV as well as contrast injection. The most used second-generation cryocatheter sprays liquid nitrogen to the distal face of the balloon through equatorial jets. Ablation is realized through the delivery of pressurized cryorefrigerant from an external console to the catheter tip via an ultrafine injection tube. Cryoablation lesion formation is based on convective cooling, whereby the cryorefrigerant absorbs heat from the surrounding myocardium, resulting in cellular damage by ice crystal formation and ischemic cell death (36). Efficacy of cryoballoon ablation for paroxysmal AF ranges from 65% to 80% at 12-month follow-up and recently it has shown to be superior to AAD administration as first-line therapy for the prevention of atrial arrhythmia recurrence in patients with paroxysmal AF in two randomized trials (7,8). Compared with antiarrhythmic drugs, cryoballoon ablation reduces the progression of paroxysmal AF toward persistent forms and reduces the hospitalization rate at three year follow-up (36). Finally, in the multicenter CRYO4PERSISTENT AF trial (37), cryoballoon ablation for the treatment of persistent AF demonstrated a 61% single-procedure success at 12-month post-ablation in addition to a significant reduction in arrhythmia-related symptoms and an improvement to quality of life. Regarding safety concerns, cryoballoon ablation seems to be associated with a lower rate of pericardial effusion and cardiac tamponade, mainly due to the lack of overheating risk. On the other hand, there is a higher incidence of phrenic nerve damage, which occurs in 1.5–2% of procedures, and that is due to cold-induced large axonal loss. However, this complication seems to be very low (<1%) in a recent real-world registry utilizing the third-generation balloon (38).
Comparison between radiofrequency and cryoballoon ablation
In order to compare the performance of the RF ablation with that of the cryoballoon ablation in a large population of patients with paroxysmal AF, the outcomes of patients undergoing prospective random assignment to RF or cryoballoon ablation were analyzed. The FIRE AND ICE trial (39) randomized 762 patients with drug-refractory paroxysmal AF. After the mean 18-month follow-up, no differences with respect to the efficacy were found; besides, both methods were comparable in terms of safety. Even if repeated ablations, cardioversions and cardiovascular rehospitalizations during the follow-up were not part of the main endpoints of the original trial, an intention-to-treat analysis showed that the cryoballoon ablation group had significantly fewer events during the follow-up, and both patient groups improved in quality-of-life scores after AF ablation (40). On the other hand, cryoballoon ablation was associated with significantly higher radiation exposure. Finally, when patients with re-ablation were analyzed, patients originally treated with cryoablation had also significantly fewer reconnected PVs (41). This issue may raise concern for RF catheter instability in certain left atrial regions; however, in the FIRE AND ICE trial a contact-force catheter was used in less than one third of the patients in the RF group. Moreover, in a recent multicenter, randomized, single-blinded trial, RF ablation and two different regimens of cryoballoon ablation resulted in no difference at one year efficacy endpoint, with greater than 98% burden reduction as assessed by continuous cardiac rhythm monitoring (42). In conclusion, we do not yet have data reporting the superiority of one source of energy, and the source choice depends on center availability and operator experience; however, more robust data are currently available in the literature regarding the outcomes of cryoballoon ablation as first-line rhythm control therapy (7,8,43).
The most representative randomized clinical trials on radiofrequency catheter ablation or cryoablation versus antiarrhythmic drugs in patients with AF are reported in Tables 1,2.
Table 1
| Study [year] | Intervention/control | ADT in control arm | Included cohort | Ablation catheter | Lesion set | Follow-up months | Main findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| Oral et al. [2006] | 77/69 | Amiodarone | Symptomatic PeAF | 8-mm NaviStar (Biosense Webster) | PVI + posterior box or roof line + mitral isthmus line | 12 | ↑ recurrence-free survival (74% vs. 58%, P=0.05); 77% crossover from control group to intervention group | (13) |
| APAF study [2006] | 99/99 | Flecainide, sotalol or amiodarone | Symptomatic drug resistant PAF | NaviStar ThermoCool (Biosense Webster) or 3.5-mm Cool-Path (St. Jude Medical) | PVI + posterior box or roof line + mitral isthmus line | 12 | ↑ recurrence-free survival (86% vs. 22%, P<0.001); ↓ cardiovascular hospitalization (P<0.01) | (14) |
| A4 study [2008] | 53/59 | Amiodarone, quinidine, disopyramide, flecainide, propafenone, cibenzoline, dofetilide, and/or sotalol. | Symptomatic PAF | NaviStar ThermoCool (Biosense Webster) or 4-mm Navistar (Biosense Webster) | PVI ± additional substrate ablation | 12 | ↑ recurrence-free survival (89% vs. 23%, P<0.001); ↑ QoL; ↑ symptom score; ↑ exercise test | (15) |
| ThermoCool AF [2010] | 106/61 | Dofetilide, flecainide, propafenone, sotalol, or quinidine | Symptomatic drug resistant PAF | NaviStar ThermoCool (Biosense Webster) | PVI ± additional substrate ablation | 9 | ↑ recurrence-free survival (66% vs. 16%, P<0.001); ↑ QoL; ↓ 30-day major adverse event (4.9% vs. 8.8%) | (16) |
| MANTRA-PAF [2012] | 146/148 | Flecainide, propafenone or amiodarone | Symptomatic drug naïve PAF | NaviStar ThermoCool (Biosense Webster) or 8-mm NaviStar (Biosense Webster) | PVI + roof line | 24 | ↑ recurrence-free survival (85% vs. 71%, P=0.004); 36% crossover from control group to intervention group | (6) |
| RAAFT-2 [2014] | 66/61 | Not specified | Symptomatic drug naïve PAF | Not specified | PVI + additional substrate ablation (operator discretion) | 24 | ↑ recurrence-free survival (72% vs. 54%, P=0.02); 43% crossover from control group to intervention group | (17) |
| CAMTAF [2014] | 26/24 | Not specified | PeAF, NYHA II–IV, LVEF <50% | Not specified | PV antrum isolation + CFAE ± roof or mitral isthmus line |
6 | ↑ mean LVEF (40%±12% vs. 31%±13%, P=0.015); ↑ peak oxygen consumption; better Minnesota questionnaire score | (18) |
| CASTLE-AF [2018] | 179/184 | Not specified | AF, NYHA II−IV, LVEF <35%, ICD | Not specified | PVI ± additional substrate ablation | 38 | ↓ composite of death or hospitalization for HF decompensation (28.5% vs. 44.6%, P=0.007) | (19) |
| CABANA [2019] | 1108/1096 | Not specified | Symptomatic AF, CHA2DS2-VASc ≥1 | Not specified | PVI ± additional substrate ablation | 48 | = composite of death, stroke, bleeding, or cardiac arrest (8% vs. 9.2%, P=0.3); ↓ AF recurrence (P<0.01) | (20) |
AF, atrial fibrillation; ADT, antiarrhythmic drug therapy; PeAF, persistent atrial fibrillation; PVI, pulmonary vein isolation; QoL, quality of life; PAF, paroxysmal atrial fibrillation; NYHA, New York Heart Association; CFAE, complex fractionated atrial electrogram; LVEF, left ventricle ejection fraction; ICD, Implantable Cardioverter-Defibrillator; HF, heart failure.
Table 2
| Study [year] | Intervention/control | ADT in control arm | Included cohort | Ablation catheter | Lesion set | Follow-up months | Main findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| STOP AF pivotal [2013] | 163/ 82 | Flecainide, propafenone, or sotalol | 78% PAF and 22% PeAF | Arctic front (Medtronic) | PVI | 12 | ↑ recurrence-free survival (69.9% vs. 7.3%, P<0.001); ↑ QoL; 3.1% PV stenosis, 1.5% 12-month phrenic nerve palsy | (21) |
| Cryo-FIRST [2021] | 107/111 | Class I or III | Symptomatic drug naïve PAF | Arctic front advance (Medtronic) | PVI | 12 | ↑ recurrence-free survival (82.2% vs. 67.6%, P=0.01); ↓ incidence rate of symptomatic palpitation | (22) |
| STOP AF First [2021] | 104/99 | Class I or III | Symptomatic drug naïve PAF | Arctic front advance (Medtronic) | PVI | 12 | ↑ recurrence-free survival (75% vs. 45%, P<0.001); ↑ QoL; = adverse event rate (14% vs. 14%) | (7) |
| EARLY-AF [2021] | 154/149 | Not specified | Symptomatic drug naïve PAF | Arctic front advance (Medtronic) | PVI | 12 | ↑ recurrence-free survival (57.1% vs. 32.2%, P<0.001); = adverse event rate (3.2% vs. 4.0%) | (8) |
AF, atrial fibrillation; ADT, antiarrhythmic drug therapy; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; PVI, pulmonary vein isolation; QoL, quality of life; Cryo, cryoablation.
Laser balloon ablation
Recently, the visually-guided laser balloon (VGLB) has been introduced as a new technology for single-shot PVI in patients with AF. It permits a direct visualization of the target atrial tissue during ablation by incorporating an endoscope located at the proximal end of the balloon. Clinical studies have demonstrated that the laser balloon is highly effective in creating transmural and durable lesions (44). The last generation of VGLB catheters has been equipped with a more compliant balloon for favorable PV occlusion and a robotically motor-driven continuous ablation mode. The lack of real-time recording system of intracardiac electrogram compared to cryoballoon techniques is counterbalanced with more possibilities to better engage different sizes and shapes of PVs using tailored inflation of the balloon. Its safety is comparable to cryoballoon and attention should be paid in order to prevent oesophageal lesions (using temperature probe) and phrenic nerve lesions.
Pulsed field electroporation
Pulsed field electroporation is a new approach that uses a non-thermal energy. The pulsed field ablation (PFA) uses sub-second electric fields to create microscopic pores in cell membranes, a process called electroporation, which produces cell necrosis selectively in the myocardium. One of the most interesting aspects of this new ablation source is that different tissues have different target thresholds, and cardiomyocytes having the lowest one. This characteristic allows for modulation of PFA to preferentially deliver myocardial damage, sparing extracardiac structures such as the esophagus or peripheral nerves that have higher thresholds. The 12-month outcomes in three multicenter study including 121 patients with paroxysmal AF have been recently published (45). PV remapping was performed to seek lesion durability. Reconnection rate was 15% but decreased to 4% in patients treated with optimized biphasic energy PFA waveform. In these patients, arrhythmia-free survival was 85% at 1 year.
These promising results have raised the expectations for PFA. However, some aspects of PFA should still be clarified. First, catheter design should be improved to minimize the amount of ablation lesion. So far, the good results in terms of lesion durability are counterbalanced by a significant amount of atrial tissue being irreversibly injured. As a consequence, macro-reentrant atrial tachycardias that have its critical isthmus linked to the PFA lesion are not infrequent. Second, new complications specifically related with this energy source are being described with the spreading of this approach, for example, coronary arterial spasm (46). Finally, it is unknown if the lack of autonomic modulation by using a myocardium selective source could influence the long-term efficacy of the ablation. In conclusion, further studies are needed to corroborate preliminary data as well as to confirm the safety of this ablation source, however, PFA could be a game-changer in catheter ablation of AF.
Extra-pulmonary veins ablation
Linear, rotor, complex fractionated electrograms and extra-pulmonary vein trigger ablation
Despite the technological innovations of the recent years, the efficacy of AF ablation in cases of persistent and long-standing persistent AF remains lower compared to those reported for paroxysmal AF. In a recent meta-analysis (10), PVI for persistent AF ablation achieved an arrhythmia-free survival rate of 66.7% at 12-month. With the objective to improve AF ablation efficacy, especially in patient with persistent AF, more extensive ablation beyond the PVI has been proposed. Unfortunately, no strategy has yet generated enough evidence to replace PVI as the standard of care during the first ablation procedure and the studies report mixed results. Ablation strategies combining PVI with additional substrate ablation, such as either linear ablation, complex fractionated atrial electrograms (CFAEs) ablation, or MRI-guided fibrosis ablation, failed to demonstrate better outcomes than PVI-only approach (11,12).
Mapping and ablating extra-PV focal triggers has been proposed in addition to the PVI. Numerous anatomical structures have been suggested as possible non-PV triggers for AF induction: the left atrial appendage (LAA), the LA posterior wall, the superior vena cava, amongst others. However, this approach has practical limitations because the induction of atrial ectopy in the electrophysiology lab can be challenging, especially if ablation is performed under sedation or general anesthesia. Besides, even if atrial ectopy is provoked, the relationship between the ectopic atrial focus and AF occurrences usually remains unproven. In a large observational series of 7,129 patients undergoing CA for AF, the presence of a LAA trigger was documented in only twenty-one patients (0.3%) (47). In the BELIEF trial (48), LAA isolation has shown to improve the efficacy of the ablation in patients with long-standing persistent AF. However, this strategy was associated with an unreasonable risk of stroke, especially in those patients in whom anticoagulation was discontinued during the follow-up. Remarkable practical issues of this approach that should be mentioned include that LAA electrical isolation is technically challenging, requiring a high amount of RF delivery, is associated with a high reconnection rate even for an experienced operator, and generally also requires LAA occlusion. The results of the recently published VENUS trial suggest that the addition of vein of Marshall ethanol infusion to PVI improves the efficacy of CA in patients with persistent AF (49). Further research is needed to confirm these results and, specially, to assess the safety of this approach.
CFAEs are defined as complex fractionated potentials or low-voltage electrograms with a short cycle length of <120 ms over a ten second period; however, the endpoints of CFAEs ablation are not clearly defined, and in the Alster-Lost-AF randomized trial, PVI plus additional CFAEs ablation demonstrated no clinical benefit over the classic PVI-only approach (50).
The roof line, the mitral isthmus line and the posterior wall isolation (PWI) are frequent linear ablation approaches for substrate modification in patients with persistent AF. However, it is difficult to achieve a durable and complete bidirectional conduction block across these lines. Besides, these strategies can result in a higher occurrence of atrial tachycardia and atypical atrial flutter due to reentry around the ablation lesions or through re-conduction across the previous line. In the same way, achieving a complete PWI can be challenging, owing to associated oesophageal heating. Studies regarding PWI efficacy showed mixed results, not confirming an incremental benefit of PWI (51).
A recent randomized study including 324 patients with persistent AF showed better outcomes by adding LA low-voltage areas ablation to PVI isolation (52). In the same vein, after the initial promising results of additional magnetic resonance-guided LA fibrosis ablation, the results of the DECAAF-II trial concluded that this approach is not superior to PVI alone in patients with persistent AF (12).
It was reported in literature that high-frequency rotors and focal impulses could be important for sustaining AF (53). This is of particular interest in patients with long-standing persistent AF. A range of technologies, such as high-density basket mapping catheters, have been used to identify and to map this high-frequency rotational activity. However, preliminary findings of the prospective randomized REAFFIRM trial reported no reduction in the rate of recurrent AF when rotor ablation was performed in addition to PVI in a cohort of patients with persistent AF. In the electrophysiological practice, an extensive ablation approach aimed to eliminate CFAEs and/or rotors is demanding and could also have pro-arrhythmic effects. Though, the benefit of these approaches should still be proven. There is an ongoing trial with the purpose of re-evaluating the additional benefit of rotational/focal activity ablation in addition to PVI compared with PVI-alone and PVI plus posterior wall isolation (STAR-AF 3 trial, NCT04442113).
Cardioneuroablation
The autonomic nervous system is an important factor in AF occurrence. It is well known that parasympathetic activation contributes to the onset and the perpetuation of AF, especially but not only, in patients with predominantly “vagal” AF. Autonomic imbalance causes the shortening of the atrial effective refractory periods and increases ectopy, especially arising from the pulmonary vein myocytes that have a shorter action potential duration and a greater sensitivity to both cholinergic and adrenergic stimulation. As a consequence, ganglionated plexuses on the epicardial surface of the atrium has become a target for ablation. Pappone et al. initially described that patients who underwent PVI and in whom vagal reflexes were completely abolished after ablation had less AF recurrences during follow-up (54). Ganglionated plexus ablation in addition to PVI seems to significantly improve freedom from atrial tachycardia/AF recurrences with respect to a PVI-only approach (55). However, which is the best method to localize these plexuses and how many plexuses should be ablated are still matter of debate.
Finally, renal denervation has recently emerged as a complement to PVI in AF therapy. In the ERADICATE-AF randomized clinical trial (56), 302 patients with AF and hypertension were randomized to PVI or renal denervation plus PVI; freedom from AF, flutter or tachycardia at 12-month was 57% and 72%, respectively (P=0.006).
Illustrations of point-by-point and one-shot approaches for AF ablation are shown in Figures 1,2.
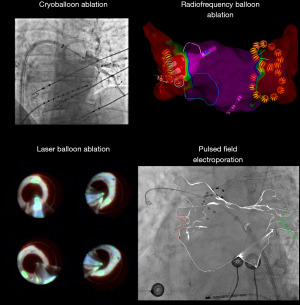
Hybrid ablation
Endocardial CA can be combined with minimally invasive thoracoscopic epicardial ablation. Hybrid ablation (HA) for AF consists of a Cox-Maze lesion set applied via minimally invasive thoracoscopic approach followed by CA, which treats gaps in the lesion set and targets any additional atrial re-entrant circuits. Recent studies reported variations regarding the energy sources used, the surgical approach, the timing of the surgical and catheter components of the procedure, the ablation lesion set applied, the management of the LAA, and the medical management of these patients. A recent meta-analysis (57) showed that in a cohort of patients with persistent AF, HA was superior to conventional ablation in maintaining sinus rhythm (71% vs. 50%, P<0.001), but on the other hand, HA had a significantly higher complication rate. The recently published CONVERGE trial (Convergence of Epicardial and Endocardial Ablation for the Treatment of Symptomatic Persistent AF) is a prospective, multicenter, randomized controlled trial (58). One hundred and fifty-three patients with persistent and long-standing persistent AF and indication for an ablation procedure were randomized to CA vs. convergent HA. This trial was hailed as a potential breakthrough, demonstrating superiority of HA over CA. The downside was that major adverse events occurred in 8% of the HA arm and 0% of the CA arm. The real need of an invasive approach in patients with persistent AF, which is often only mildly symptomatic, should be carefully evaluated, and appropriate patient selection is fundamental.
Zero- or nearly zero-fluoroscopy approaches
Historically, all AF ablations were performed under fluoroscopy guidance. Recently, the awareness of risk associated with radiation exposure to patients and professional staff has significantly increased. Several studies demonstrated the direct relationship between the radiation dose and the lifelong risk of both deterministic and stochastic side effects. An important effort was therefore made to minimize radiation exposure, as recommended by the American College of Cardiology in the ALARA statement: keep the radiation “as low as reasonably achievable”. Technological advances such as 3D navigation systems have enabled the reduction of radiation use, even allowing a nearly zero-fluoroscopy approach (5). Concerns regarding trans-septal access have limited the fluoro-less approach for LA ablations. In this setting, there is growing evidence that the use of the electro-anatomical maps and the navigation system combined with intracardiac echocardiography to guide trans-septal puncture allows reduction or even elimination of fluoroscopy (59), with the downside of the need for an additional venous puncture, a trained cardiologist and an increase in procedural cost. Moreover, a recent study reported a series of 111 patients demonstrating the procedural feasibility of a transesophageal echocardiography-guided CA for AF with a complete or near-complete avoidance of radiological exposure (60).
A representative case of zero-fluoroscopy approach for AF radiofrequency ablation was reported and explained via audio recording in the presentation (Video 1).
Conclusions
CA is a widespread and fundamental therapeutic strategy for rhythm-control in AF patients. Advances in technologies and strategies have increasingly led to an improvement in efficacy, safety and a reduction in procedural requirements. The innovations and new evidence are continuing to expand the indications of CA as first-line therapy in symptomatic patients and now also as an early rhythm-control strategy in asymptomatic patients. Despite the progress in CA techniques, long-term outcomes after a single procedure remain suboptimal in patients with persistent and long-standing persistent AF. PVI remains the therapeutic cornerstone of CA for all AF types; further investigations are required to determine new appropriate strategies when PVI-alone is insufficient. Ongoing research may offer new therapeutic targets and options for AF patients with difficult-to-treat arrhythmia.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. [Crossref] [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [Crossref] [PubMed]
- Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101:406-26.
- Haïssaguerre M, Shah DC, Jaïs P, et al. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation 2000;102:2463-5. [Crossref] [PubMed]
- Pappone C, Oreto G, Lamberti F, et al. Catheter ablation of paroxysmal atrial fibrillation using a 3D mapping system. Circulation 1999;100:1203-8. [Crossref] [PubMed]
- Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587-95. [Crossref] [PubMed]
- Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N Engl J Med 2021;384:305-15. [Crossref] [PubMed]
- Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N Engl J Med 2021;384:316-24. [Crossref] [PubMed]
- El Haddad M, Taghji P, Phlips T, et al. Determinants of Acute and Late Pulmonary Vein Reconnection in Contact Force-Guided Pulmonary Vein Isolation: Identifying the Weakest Link in the Ablation Chain. Circ Arrhythm Electrophysiol 2017;10:e004867. [Crossref] [PubMed]
- Voskoboinik A, Moskovitch JT, Harel N, et al. Revisiting pulmonary vein isolation alone for persistent atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm 2017;14:661-7. [Crossref] [PubMed]
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812-22. [Crossref] [PubMed]
- Marrouche NF, Wazni O, McGann C, et al. Effect of MRI-Guided Fibrosis Ablation vs Conventional Catheter Ablation on Atrial Arrhythmia Recurrence in Patients With Persistent Atrial Fibrillation: The DECAAF II Randomized Clinical Trial. JAMA 2022;327:2296-305. [Crossref] [PubMed]
- Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med 2006;354:934-41. [Crossref] [PubMed]
- Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006;48:2340-7. [Crossref] [PubMed]
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498-505. [Crossref] [PubMed]
- Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333-40.
- Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692-700. [Crossref] [PubMed]
- Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31-8. [Crossref] [PubMed]
- Marrouche NF, Brachmann J, Andresen D, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med 2018;378:417-27. [Crossref] [PubMed]
- Packer DL, Mark DB, Robb RA, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019;321:1261-74. [Crossref] [PubMed]
- Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713-23. [Crossref] [PubMed]
- Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace 2021;23:1033-41. [Crossref] [PubMed]
- Kirchhof P, Camm AJ, Goette A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med 2020;383:1305-16. [Crossref] [PubMed]
- Al-Khatib SM, Allen LaPointe NM, Chatterjee R, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med 2014;160:760-73. [Crossref] [PubMed]
- Packer DL, Piccini JP, Monahan KH, et al. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure: Results From the CABANA Trial. Circulation 2021;143:1377-90. [Crossref] [PubMed]
- Hong KL, Borges J, Glover B. Catheter ablation for the management of atrial fibrillation: current technical perspectives. Open Heart 2020;7:e001207. [Crossref] [PubMed]
- Parameswaran R, Al-Kaisey AM, Kalman JM. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol 2021;18:210-25. [Crossref] [PubMed]
- Taghji P, El Haddad M, Phlips T, et al. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins With Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibrillation: A Pilot Study. JACC Clin Electrophysiol 2018;4:99-108. [Crossref] [PubMed]
- Del Monte A, Almorad A, Pannone L, et al. Pulmonary vein isolation with the radiofrequency balloon catheter: a single centre prospective study. Europace 2023;25:896-904. [Crossref] [PubMed]
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent Atrial Fibrillation Ablation With or Without Contact Force Sensing. J Cardiovasc Electrophysiol 2017;28:483-8. [Crossref] [PubMed]
- Zucchelli G, Sirico G, Rebellato L, et al. Contiguity Between Ablation Lesions and Strict Catheter Stability Settings Assessed by VISITAG(TM) Module Improve Clinical Outcomes of Paroxysmal Atrial Fibrillation Ablation - Results From the VISITALY Study. Circ J 2018;82:974-82. [Crossref] [PubMed]
- Duytschaever M, De Pooter J, Demolder A, et al. Long-term impact of catheter ablation on arrhythmia burden in low-risk patients with paroxysmal atrial fibrillation: The CLOSE to CURE study. Heart Rhythm 2020;17:535-43. [Crossref] [PubMed]
- Di Biase L, Monir G, Melby D, et al. Composite Index Tagging for PVI in Paroxysmal AF: A Prospective, Multicenter Postapproval Study. JACC Clin Electrophysiol 2022;8:1077-89. [Crossref] [PubMed]
- Reddy VY, Grimaldi M, De Potter T, et al. Pulmonary Vein Isolation With Very High Power, Short Duration, Temperature-Controlled Lesions: The QDOT-FAST Trial. JACC Clin Electrophysiol 2019;5:778-86. [Crossref] [PubMed]
- Schoene K, Arya A, Grashoff F, et al. Oesophageal Probe Evaluation in Radiofrequency Ablation of Atrial Fibrillation (OPERA): results from a prospective randomized trial. Europace 2020;22:1487-94. [Crossref] [PubMed]
- Andrade JG. Cryoballoon ablation for pulmonary vein isolation. J Cardiovasc Electrophysiol 2020;31:2128-35. [Crossref] [PubMed]
- Boveda S, Metzner A, Nguyen DQ, et al. Single-Procedure Outcomes and Quality-of-Life Improvement 12 Months Post-Cryoballoon Ablation in Persistent Atrial Fibrillation: Results From the Multicenter CRYO4PERSISTENT AF Trial. JACC Clin Electrophysiol 2018;4:1440-7. [Crossref] [PubMed]
- Földesi C, Misiková S, Ptaszyński P, et al. Safety of cryoballoon ablation for the treatment of atrial fibrillation: First European results from the cryo AF Global Registry. Pacing Clin Electrophysiol 2021;44:883-94. [Crossref] [PubMed]
- Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med 2016;374:2235-45. [Crossref] [PubMed]
- Kuck KH, Fürnkranz A, Chun KR, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37:2858-65. [Crossref] [PubMed]
- Kuck KH, Albenque JP, Chun KJ, et al. Repeat Ablation for Atrial Fibrillation Recurrence Post Cryoballoon or Radiofrequency Ablation in the FIRE AND ICE Trial. Circ Arrhythm Electrophysiol 2019;12:e007247. [Crossref] [PubMed]
- Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019;140:1779-88. [Crossref] [PubMed]
- Zucchelli G, Chun KRJ, Khelae SK, et al. Impact of first-line cryoablation for atrial fibrillation on healthcare utilization, arrhythmia disease burden and efficacy outcomes: real-world evidence from the Cryo Global Registry. J Interv Card Electrophysiol 2023;66:711-22. [Crossref] [PubMed]
- Dukkipati SR, Neuzil P, Skoda J, et al. Visual balloon-guided point-by-point ablation: reliable, reproducible, and persistent pulmonary vein isolation. Circ Arrhythm Electrophysiol 2010;3:266-73. [Crossref] [PubMed]
- Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation: 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614-27. [Crossref] [PubMed]
- Reddy VY, Petru J, Funasako M, et al. Coronary Arterial Spasm During Pulsed Field Ablation to Treat Atrial Fibrillation. Circulation 2022;146:1808-19. [Crossref] [PubMed]
- Al Rawahi M, Liang JJ, Kapa S, et al. Incidence of Left Atrial Appendage Triggers in Patients With Atrial Fibrillation Undergoing Catheter Ablation. JACC Clin Electrophysiol 2020;6:21-30. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J Am Coll Cardiol 2016;68:1929-40. [Crossref] [PubMed]
- Valderrábano M, Peterson LE, Swarup V, et al. Effect of Catheter Ablation With Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation: The VENUS Randomized Clinical Trial. JAMA 2020;324:1620-8. [Crossref] [PubMed]
- Fink T, Schlüter M, Heeger CH, et al. Stand-Alone Pulmonary Vein Isolation Versus Pulmonary Vein Isolation With Additional Substrate Modification as Index Ablation Procedures in Patients With Persistent and Long-Standing Persistent Atrial Fibrillation: The Randomized Alster-Lost-AF Trial (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation). Circ Arrhythm Electrophysiol 2017;10:e005114. [Crossref] [PubMed]
- Thiyagarajah A, Kadhim K, Lau DH, et al. Feasibility, Safety, and Efficacy of Posterior Wall Isolation During Atrial Fibrillation Ablation: A Systematic Review and Meta-Analysis. Circ Arrhythm Electrophysiol 2019;12:e007005. [Crossref] [PubMed]
- Huo Y, Gaspar T, Schönbauer R, et al. Low-Voltage Myocardium-Guided Ablation Trial of Persistent Atrial Fibrillation. NEJM Evid 2022;1: [Crossref]
- Jalife J. Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol 2003;14:776-80. [Crossref] [PubMed]
- Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109:327-34. [Crossref] [PubMed]
- Kampaktsis PN, Oikonomou EK, Y, Choi D, et al. Efficacy of ganglionated plexi ablation in addition to pulmonary vein isolation for paroxysmal versus persistent atrial fibrillation: a meta-analysis of randomized controlled clinical trials. J Interv Card Electrophysiol 2017;50:253-60. [Crossref] [PubMed]
- Steinberg JS, Shabanov V, Ponomarev D, et al. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA 2020;323:248-55. [Crossref] [PubMed]
- van der Heijden CAJ, Vroomen M, Luermans JG, et al. Hybrid versus catheter ablation in patients with persistent and longstanding persistent atrial fibrillation: a systematic review and meta-analysis†. Eur J Cardiothorac Surg 2019;56:433-43. [Crossref] [PubMed]
- DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ Arrhythm Electrophysiol 2020;13:e009288. [Crossref] [PubMed]
- Bulava A, Hanis J, Eisenberger M. Catheter Ablation of Atrial Fibrillation Using Zero-Fluoroscopy Technique: A Randomized Trial. Pacing Clin Electrophysiol 2015;38:797-806. [Crossref] [PubMed]
- Falasconi G, Penela D, Soto-Iglesias D, et al. A standardized stepwise zero-fluoroscopy approach with transesophageal echocardiography guidance for atrial fibrillation ablation. J Interv Card Electrophysiol 2022;64:629-39. [Crossref] [PubMed]







