Atrial fibrillation symptom reduction and improved quality of life following the hybrid convergent procedure: a CONVERGE trial subanalysis
Introduction
Patients with untreated atrial fibrillation (AF) experience significant impairments in their quality of life (QOL) (1,2) that can occur even when AF is otherwise, believed to be asymptomatic (3). Several factors may influence the QOL of a patient experiencing AF including age, sex, time since onset, and cardiovascular and pulmonary comorbidities (4). Obesity, anxiety, and depression in patients with AF have been demonstrated as associated factors resulting in lower QOL (5). In addition, lower QOL is associated with greater AF burden and longer duration of AF episodes (6). Improvement in symptoms of AF and AF burden therefore may increase patients’ QOL.
The 2017 Heart Rhythm Society (HRS) consensus statement considers the reduction of patient symptoms and QOL improvement the primary reasons to indicate catheter ablation (CA) (3). CA to isolate the pulmonary veins (PV) is effective in the treatment of patients with paroxysmal AF (i.e., AF that terminates within 7 days) (7-9). Recent investigational device exemption (IDE) clinical trials reported favorable outcomes following the use of cryoballoon and radiofrequency CA in the treatment of patients with early PersAF less than 6 months (10) and PersAF less than 12 months (11), with improvements in AF-related symptoms and QOL in post-procedure follow-up (10,12). Patients with persistent AF (PersAF) and longstanding PersAF (LSPAF) may tolerate their symptoms better than patients with paroxysmal AF because of living with the condition for a prolonged period. With treatment that reestablishes sinus rhythm in PersAF and LSPAF patients, improvement in AF symptoms, although less than what may be achieved in paroxysmal AF or early PersAF, may be possible and beneficial to their QOL (3).
We recently reported on the use of the Hybrid AF Convergent (HC) in PersAF and LSPAF in the prospective, multicenter, randomized controlled CONVERGE IDE clinical trial (13). The HC procedure combines epicardial ablation performed by a cardiothoracic surgeon and endocardial CA performed by an electrophysiologist to achieve PV and left atrial posterior wall isolation with the goal of creating durable, transmural lesions while minimizing the risk of collateral thermal injury. In CONVERGE, 153 patients were randomized and treated 2:1 via the HC procedure or conventional CA (13). As previously reported, 67.7% (67/99) of evaluable patients who received HC achieved freedom from AF/atrial flutter (AFL)/atrial tachycardia (AT) through 12 months compared with 50.0% (25/50) of evaluable patients who received CA off new anti-arrhythmic drugs (AADs) or increased dose of previously failed/intolerant AADs. For patients off AADs, 53.5% (53/99) of patients with HC achieved freedom from AF/AFL/AT through 12 months versus 32.0% (16/50) of patients with CA. The primary safety endpoint was met. In a subanalysis of 65 LSPAF patients (42.5% of the initial 153 patients), freedom from AF/AFL/AT off new or increased dose of previously failed/intolerant AADs through 12 months was 65.8% (25/38) in the Hybrid Convergent arm and 37.0% (10/27) in the CA arm (P=0.022). For the LSPAF subgroup, the rates off AADs were 52.6% (20/38) in the Hybrid Convergent group and 25.9% (7/27) in the CA group (P=0.031) (14). In the overall PersAF and LSPAF cohort, the 18-month effectiveness outcome of ≥90% AF burden reduction was met in 74% (53/72) of the HC group and 55% (23/42) in the CA group, using 7-day Holter monitoring (13). In the LSPAF subgroup, 73% (27/37) in the Hybrid Convergent group and 36% (9/25) in the CA group had ≥90% AF burden reduction (P=0.004) at 18 months (14). With the knowledge of the safety and effectiveness of the HC procedure, the objective of the present analysis is to report on the impact of HC ablation on AF symptoms and QOL of patients in the CONVERGE clinical trial.
Methods
Study design
The methods of the CONVERGE trial (ClinicalTrials.gov Identifier: NCT01984346) and complete inclusion/exclusion criteria have been previously published (13,15). Enrolled patients were randomly assigned 2:1 to receive HC or endocardial CA. The CONVERGE trial was conducted in accordance with the Declaration of Helsinki. The study was approved by Institutional Review Board or ethics committee and patient informed consents were obtained.
Patients
Eligible patients from 27 sites (25 United States and 2 United Kingdom) participated in the CONVERGE trial and were aged 18–80 years, diagnosed with symptomatic PersAF or LSPAF that was refractory or intolerant to at least 1 class I/III AAD, and had a left atrium diameter of ≤6.0 cm.
Intervention
The interventions in both arms have been described elsewhere and are briefly summarized here (15). For HC ablation, the epicardial and endocardial procedures were performed in a single setting by a cardiothoracic surgeon and electrophysiologist, respectively. Epicardial linear lesions were created across the posterior left atrial wall using a unipolar radiofrequency device (EPi-Sense, AtriCure, Inc., Mason, OH, USA) through a transdiaphragmatic or subxiphoid endoscopic approach. Endocardial ablation was performed with a commercially available endocardial radiofrequency catheter to complete PV isolation, connect any gaps, and create a cavotricuspid isthmus (CTI) line. For the CA arm, a commercially available endocardial radiofrequency catheter was used to isolate the PVs and create a roof line and CTI line.
Data collection
For the previously published analysis, in-person follow-up visits were performed at 7 days, and 1, 3, 6, 12, and 18 months, and included an electrogram and review of medications and adverse events (13). The 6- and 12-month visits included 24-hour Holter monitoring, and the 18-month visit included 7-day Holter monitoring. The trial remains ongoing and includes planned phone follow-up at 2, 3, 4, and 5 years. For the present analysis, presence of AF symptoms was assessed using the Atrial Fibrillation Severity Scale (AFSS) at baseline and 12 months following treatment. The AFSS is a 19-item self-administered questionnaire developed to capture subjective and objective ratings of AF-related symptoms, health care utilization, and AF disease burden, including frequency, duration, and severity of episodes (1,16). The AFSS Composite is scored from 3 to 30 and higher values represent greater burden as follows: Overall Symptom is scored as 1 = less severe to 35 = more severe; Overall Patient-Perceived Severity is scored as 1 = not at all severe to 10 = extremely severe; Global Well-Being is scored as 1 = worst possible life to 10 = best possible life; Atrial Fibrillation Frequency is scored as 1= continuously to 11 = less than once a year; and Atrial Fibrillation Duration is scored as 1 = continuously to 8 = a few minutes. QOL was assessed using the 36-Item Short Form Health Survey (SF-36) at baseline and 12 months following treatment. The SF-36 is a validated, self-administered generic survey not specific for AF that assesses two component scores [Physical Component Score (PCS) and Mental Component Score (MCS)] including eight total domains: physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health (17). SF-36 values range from 0 to 100 and higher values represent better QOL. A 5-point change in scores is considered “clinically and socially” relevant (18). Class I/III AAD use was assessed at baseline and then after a 3-month blanking period through 12 and 18 months following treatment.
Statistical analysis
Statistical analysis of change in AAD use was performed using McNemar’s tests. Statistical analysis of change in AFSS and SF-36 scores within group were performed using paired t-tests and between groups using Analysis of Covariance (ANCOVA). All P values reported were two sided with a significance threshold of 0.05 and without multiplicity adjustment. Statistical analysis was provided by NAMSA (Northwood, OH, USA) using SAS v 9.4 (Cary, NC, USA).
Results
Change in AF symptoms from baseline to 12 months following HC
In CONVERGE, a total of 153 patients were treated for AF with either HC (n=102) or CA (n=51). Baseline patient characteristics were previously reported, with significant differences in the two cohorts with respect to males, 78% males in HC group and 53% in CA group (P=0.0016). This difference did not impact the overall study results. No other baseline parameters were significantly different between groups, including age (63.7±9.6 vs. 65.1±6.7 years HC and CA, respectively) (13). Of the 153 treated patients, 65 (42%) patients had LSPAF, including 38 patients treated with HC and 27 patients treated with CA. AFSS Composite and Overall Symptoms Scores significantly improved at 12 months versus baseline for the HC arm and the subgroup of HC patients with LSPAF (Figure 1). The mean change in AFSS Composite Scores from baseline to 12 months in the HC arm was −11.7 (P<0.001). In the HC LSPAF subgroup, mean change in AFSS Composite Score from baseline to 12 months was −13.0 (P<0.001). Similar decreases in AFSS Composite Scores from baseline to 12 months were observed in the overall and LSPAF populations who received CA (Figure S1). Mean changes in AFSS Overall Symptom Scores were −10.1 and −9.9 from baseline to 12 months for the overall and LSPAF populations, respectively, who received HC (P<0.001 for both; Figure 1). Changes in AFSS Overall Symptom Scores were significantly greater after HC than after CA (Figure S1).
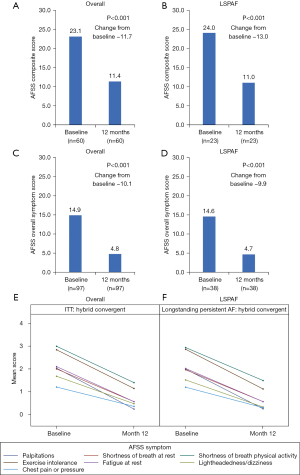
From baseline, reductions in the AF symptoms of palpitations, shortness of breath at rest, shortness of breath during physical activity, exercise intolerance, fatigue at rest, lightheadedness/dizziness, and chest pain or pressure were observed at 12 months follow-up after the HC procedure for both the overall HC arm and in the LSPAF subgroup of the HC arm (Figure 1). Reductions in individual AF symptom scores at month 12 also occurred for patients treated with CA (Figure S2).
Change in QOL from baseline to 12 months following HC
The SF-36 MCS and PCS significantly improved at 12 months versus baseline for the HC arm and the LSPAF subgroup of HC patients (Figures 2,3). The mean change in SF-36 MCS from baseline to 12 months in the HC arm and the LSPAF subgroup was +5.6 (P<0.001 and P=0.01, respectively; Figure 2). Similar improvements in MCS from baseline to 12 months were seen in the overall and LSPAF patients treated with CA (Figure S3). The mean change in SF-36 PCS from baseline to 12 months in the HC arm was +7.3 (P<0.001; Figure 3). In the HC LSPAF subgroup, mean change in SF-36 PCS from baseline to 12 months was +7.9 (P<0.001). Improvement in PCS from baseline to 12 months was similar after HC for the overall population compared with CA, whereas improvement in PCS was significantly greater after HC than CA in the LSPAF subgroup (Figure S4).
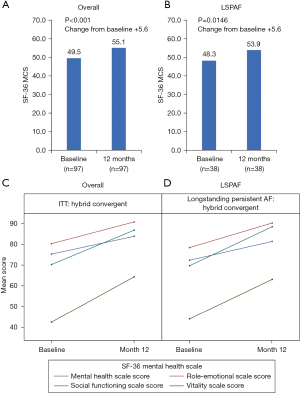

From baseline to 12 months in the HC arm, improvement in SF-36 Mental Health scales (Figure 2) and Physical Health scales (Figure 3) were observed for the overall group and for the subgroup of patients with LSPAF. The individual SF-36 Mental and Physical Health scales for patients treated with CA are shown in Figures S5,S6.
AAD use from baseline to 18 months following HC
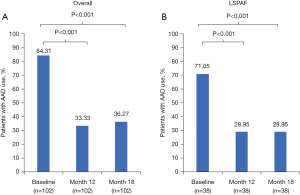
The proportion of patients in the HC arm (n=102) who used Class I /III AADs post-blanking period was significantly lower at 12 months (33.3%) and 18 months (36.3%) than baseline (84.3%; P<0.001; Figure 4). For HC patients with LSPAF (n=38), the proportions of AAD use were significantly lower at 12 and 18 months (both 29.0%) than at baseline (71.1%; P<0.001). The proportion of patients in the CA arm (n=51) who used Class I /III AADs post-blanking period through 12 months was 56.9% versus 80.4% at baseline (P=0.0105; Figure S7). In contrast, AAD use for CA patients with LSPAF (n=27) was similar at 12 months and 18 months (both 63.0%) versus baseline (66.7%, P=0.7630).
Discussion
CONVERGE was the first multicenter, randomized controlled trial to compare the effectiveness of combined epicardial and endocardial (HC) ablation to endocardial CA for the treatment of patients with PersAF and LSPAF and remains the only contemporary IDE trial thus far to include a large portion of patients with LSPAF. The data presented here demonstrate that in patients with PersAF or LSPAF, AF symptoms (i.e., the AFSS Overall Symptom Score) were significantly reduced at 12 months follow-up from baseline after the HC procedure. QOL significantly improved with HC from baseline to 12 months follow-up. AAD use was significantly lower with HC through both 12 and 18 months. Additionally, significantly greater improvements in the PCS of the SF-36 and the overall AFSS Overall Symptom score were observed from baseline to 12 months in the subset of patients with LSPAF treated with HC compared to theses outcomes in patients with LSPAF treated with CA. A significant reduction in Class I/III AAD use was observed through 12 and 18 months following HC ablation in patients with LSPAF, however AAD use was not reduced in LSPAF patients treated with CA. In assessment of all patients in the CA arm (PersAF and LSPAF), AF symptoms and QOL were improved at 12 months compared with baseline, and proportion of patients using Class I/III AADs was significantly reduced through 12 and 18 months from baseline in the CA arm.
Our findings regarding QOL, support previously published QOL data from several studies of patients with PersAF treated with various endocardial ablation strategies (10,12). In several studies, the improvements in QOL have been attributed to and associated with the reduction in AF burden (3,6,13,19). Data from the CONVERGE trial found that ≥90% AF burden reduction was experienced by 80% of patients with PersAF and LSPAF treated with HC at 12 months post-procedure compared with baseline (13), indicating a clinically meaningful reduction in AF burden. In a 2021 CAPTAF study analysis of 150 patients with paroxysmal and PersAF under continuous monitoring, greater AF burden and longer AF episodes were significantly associated with lower QOL in simple linear regression analyses (6). A subsequent multiple linear regression analysis found that only greater AF burden had a significant effect on QOL. In the STAR-AF II trial, an AF burden reduction of greater than 70% from baseline was found to be associated with higher QOL scores (19). Our CONVERGE results are in line with these findings that QOL improvements occur in parallel with substantial AF burden reduction even in the most difficult to treat cases of AF.
Although both PersAF and LSPAF patients may tolerate their symptoms better than patients with paroxysmal AF, there are substantial benefits in treatment and subsequent return to sinus rhythm (3). Although published data on QOL in LSPAF patients is limited, Bulková et al. also showed that in 126 patients with LSPAF, their QOL remained significantly greater 3 years post CA compared with 261 patients with paroxysmal AF (20). The authors concluded in patients with LSPAF the positive effect after CA was partially attributed to reduced reliance on AADs for rhythm control. The present analysis addresses lingering questions by demonstrating patients with LSPAF experience an improvement in QOL following hybrid ablation, as well as reduced AF symptoms and reduced reliance on Class I/III AADs, the latter of which provides alleviation from drug side effects. The significantly improved physical function in LSPAF following HC will likely be clinically relevant to patients.
We acknowledge the limitations imposed by the retrospective nature of our study, including the absence of empirical endocardial posterior wall ablation in the CA group. Obtaining transmural posterior wall ablation while maintaining safety can be challenging, and it is difficult to state if the outcomes would have been better in the CA arm posterior wall if silencing was allowed (13). Another limitation is the use of generic SF-36 QOL questionnaires, which were self-administered and lack the ability to determine symptoms related to AF, introducing possible bias. Lastly, the estimate of AF burden was based on frequent Holter and ECG monitoring rather than using continuous implantable monitoring.
These results add to the body of evidence that in patients with LSPAF, epicardial posterior wall ablation via the HC procedure is valuable beyond the restoration of sinus rhythm. As such, additional studies are needed to determine whether improvements in QOL and AF symptoms following HC in both PersAF and LSPAF are sustained for longer than 12 months following treatment.
Acknowledgments
The authors would like to acknowledge and thank the patients, participating study centers, and study investigators of the CONVERGE trial. Latoya M. Mitchell, PhD, CMPP, and Marcie Meador, PhD, RN, CMPP of AtriCure, Inc. provided medical writing assistance under the direction of the authors for this manuscript. Statistical analysis and data verification were performed by NAMSA (Northwood, OH) and funded by AtriCure, Inc. (Mason, OH). These data were presented in part at Heart Rhythm 2022, Apr 29–May 1, 2022, in San Francisco, CA.
Funding: The CONVERGE trial was sponsored and funded by AtriCure, Inc. (Mason, OH).
Footnote
Conflicts of Interest: J.G. reports research funding from Abbott and lecture honoraria from AtriCure. C.B. has consulted for New Cardioplegia Solutions as well as proctoring for AtriCure. F.K. has received research grants from AtriCure; he has consulted for CryoLife, Edwards, LivaNova, and Medtronic. S.R.O. has consulted for Biosense Webster and has received compensation for services from AtriCure. M.A.M. has received compensation for services from AtriCure. M.E.H. reports advisory board membership and consultant fees from Medtronic. D.T. has received compensation for services from AtriCure. J.O. has consulted for Biosense Webster and Boston Scientific and has received compensation for services from AtriCure. S.A. reports speaker bureau membership for AtriCure. A.K. reports advisory board membership and consultant fees from AtriCure. C.S. reports consultant fees and honoraria from Abbott Laboratories, AtriCure, and Medtronic. D.M.G. has received compensation for services from AtriCure. M.K. reports consultant fees from Medtronic. I.J. reports advisory board membership and consultant fees from AtriCure. F.Y. reports advisory board membership and consultant fees from AtriCure. D.B.D.L. is a consultant and speaker for AtriCure and Boston Scientific, and a consultant to Medtronic. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303-9. [Crossref] [PubMed]
- Guédon-Moreau L, Capucci A, Denjoy I, et al. Impact of the control of symptomatic paroxysmal atrial fibrillation on health-related quality of life. Europace 2010;12:634-42. [Crossref] [PubMed]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275-444. [Crossref] [PubMed]
- Randolph TC, Simon DN, Thomas L, et al. Patient factors associated with quality of life in atrial fibrillation. Am Heart J 2016;182:135-43. [Crossref] [PubMed]
- Charitakis E, Barmano N, Walfridsson U, et al. Factors Predicting Arrhythmia-Related Symptoms and Health-Related Quality of Life in Patients Referred for Radiofrequency Ablation of Atrial Fibrillation: An Observational Study (the SMURF Study). JACC Clin Electrophysiol 2017;3:494-502. [Crossref] [PubMed]
- Jansson V, Bergfeldt L, Schwieler J, et al. Atrial fibrillation burden, episode duration and frequency in relation to quality of life in patients with implantable cardiac monitor. Int J Cardiol Heart Vasc 2021;34:100791. [Crossref] [PubMed]
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014;64:647-56. [Crossref] [PubMed]
- Knight BP, Novak PG, Sangrigoli R, et al. Long-Term Outcomes After Ablation for Paroxysmal Atrial Fibrillation Using the Second-Generation Cryoballoon: Final Results From STOP AF Post-Approval Study. JACC Clin Electrophysiol 2019;5:306-14. [Crossref] [PubMed]
- Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713-23. [Crossref] [PubMed]
- Su WW, Reddy VY, Bhasin K, et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: Results from the multicenter STOP Persistent AF trial. Heart Rhythm 2020;17:1841-7. [Crossref] [PubMed]
- Mansour M, Calkins H, Osorio J, et al. Persistent Atrial Fibrillation Ablation With Contact Force-Sensing Catheter: The Prospective Multicenter PRECEPT Trial. JACC Clin Electrophysiol 2020;6:958-69. [Crossref] [PubMed]
- Natale A, Calkins H, Osorio J, et al. Positive Clinical Benefit on Patient Care, Quality of Life, and Symptoms After Contact Force-Guided Radiofrequency Ablation in Persistent Atrial Fibrillation: Analyses From the PRECEPT Prospective Multicenter Study. Circ Arrhythm Electrophysiol 2021;14:e008867. [Crossref] [PubMed]
- DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ Arrhythm Electrophysiol 2020;13:e009288. [Crossref] [PubMed]
- DeLurgio DB, Blauth C, Halkos ME, et al. Hybrid epicardial-endocardial ablation for long-standing persistent atrial fibrillation: A subanalysis of the CONVERGE Trial. Heart Rhythm O2 2023;4:111-8. [Crossref] [PubMed]
- DeLurgio DB, Ferguson E, Gill J, et al. Convergence of Epicardial and Endocardial RF Ablation for the Treatment of Symptomatic Persistent AF (CONVERGE Trial): Rationale and design. Am Heart J 2020;224:182-91. [Crossref] [PubMed]
- Dorian P, Paquette M, Newman D, et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J 2002;143:984-90. [Crossref] [PubMed]
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83.
- Ware JE, Jr., Snow KK, Kosinski M, Gandek B. SF-36 health survey: Manual and interpretation guide. The Health Institute, New England Medical Center; 1997.
- Terricabras M, Mantovan R, Jiang CY, et al. Association Between Quality of Life and Procedural Outcome After Catheter Ablation for Atrial Fibrillation: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open 2020;3:e2025473. [Crossref] [PubMed]
- Bulková V, Fiala M, Havránek S, et al. Improvement in quality of life after catheter ablation for paroxysmal versus long-standing persistent atrial fibrillation: a prospective study with 3-year follow-up. J Am Heart Assoc 2014;3:e000881. [Crossref] [PubMed]






