Obstetric considerations for aortopathy in pregnancy
Introduction
Aortopathy refers to disorders of the aorta, including aortic aneurysms and dissections. Despite its rarity, aortic dissection (AD) associated with pregnancy poses a significant risk as it can lead to potentially catastrophic events for mother and/or fetus. While there is no formal definition for what constitutes an AD associated with pregnancy, previous literature suggests a timeline which extends from conception to 6–12 weeks postpartum (1-4). AD during pregnancy can be due to genetic aortopathy, sporadic, or traumatic aortic injury (5). This review aims to provide an overview of genetic conditions associated with aortopathy, obstetric risks, and management strategies to assist in the optimization of patient care, and to highlight special clinical scenarios related to AD in pregnancy.
Incidence and significance of AD associated with pregnancy
AD associated with pregnancy occurs in 4–5 per 1,000,000 pregnancies (6) and is the third most frequent cause of maternal death from cardiovascular disease (7). Historic cohorts report maternal mortality rates for AD associated with pregnancy are as high as 30%, and fetal loss rates range from 20–50% (8). However, modern cohorts suggest mortality rates are probably closer to 6–10% maternal and 10–20% fetal (9-11).
Pregnancy increases the risk for AD 4- to 25-fold (6,12,13). The increased risk is theorized to be related to physiologic cardiovascular and hormonal changes in pregnancy that begin in the first trimester and peak in the third trimester of pregnancy (14-16). These physiologic changes include increased blood volume, cardiac output, and maternal heart rate, along with decreased arterial blood pressure and systemic vascular resistance (14-16). Cardiac output, blood volume, and heart rate peak in the third trimester around 28–30 weeks gestation. Additionally, estrogen and progesterone increase as gestation progresses. There are estrogen receptors present in the human aorta. The hormonal changes of pregnancy along with cardiovascular changes lead to increased shearing forces on vasculature during pregnancy. Histologic studies have shown changes in the integrity of larger arteries including loss of reticulin, decreased mucopolysaccharides, loss of corrugation fibers, and hypertrophy of smooth muscle in the large arteries of pregnant persons on postmortem exams (17,18).
Type and timing of AD associated with pregnancy
Type A dissections are slightly more common in pregnancy, comprising 50–60%; while type B dissections account for 40–50% of ADs during pregnancy (1,9,11,19). While type A dissections are usually associated with aortic root or ascending aortic dilation, type B ADs may occur without descending thoracic aortic dilation. The majority of Ads occur in the third trimester and postpartum period. Up to 60% of type A dissections occur in the third trimester and 40–50% in the postpartum period. Up to 50% of type B ADs occur in the postpartum period, while only 25% occur in the third trimester (1).
Risk factors for AD associated with pregnancy
Multiple risk factors for AD associated with pregnancy have been studied (Table 1). These include genetic conditions with a predisposition for AD, preexisting hypertension and hypertensive disorders of pregnancy, history of previous cardiac surgery or congenital heart disease including aortic coarctation, inflammatory vasculitis, certain infections, and exposure to cocaine (2). If one or more of these risk factors are identified, it is important for the patient to be counseled about the potential risk of AD in a pregnancy.
Table 1
| HTAD including Marfan syndrome, Loeys-Dietz syndrome, bicuspid aortic valve with aortic aneurysm, Turner syndrome, vascular Ehlers-Danlos syndrome, nsHTAD |
| Concern for HTAD and family history of aortic dissection |
| Hypertensive disorders of pregnancy and preexisting hypertension in pregnancy |
| History of previous cardiac surgery or congenital heart disease |
| Inflammatory vasculitis |
| Environment exposures (cocaine) |
HTAD, heritable thoracic aortic disease; nsHTAD, non-syndromic heritable thoracic aortic disease.
Obstetric considerations for individuals with genetic aortopathy
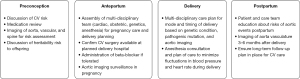
The majority of individuals who experience AD associated with pregnancy have a genetic predisposition for AD (1). People with genetic aortopathy can be further risk-stratified based on clinical characteristics including aortic root size, rapid aortic root growth >3 mm during pregnancy, and family history of individuals who have had AD (20). Beyond aortic root size, individualized risk is not able to be predicted despite having these known risk factors. In general, many individuals with known genetic aortopathies have healthy pregnancies without aortic complications, but it is important to discuss risks and have a plan of care prior to pregnancy if able. The management recommendations are divided into pre-conception, antepartum, delivery and postpartum care (Figure 1).
Pre-conception counseling
A pre-pregnancy consultation with a maternal-fetal medicine specialist or cardio-obstetrics team is recommended for all persons with genetic aortopathy to discuss the cardiovascular risks of pregnancy, heritability risks to offspring, and to allow for adjustments to any medications that are not recommended during pregnancy. A shared decision-making model should be employed to review the safety and risks of pregnancy, ultimately letting the individual determine if they want to pursue a pregnancy. It is also important to discuss alternatives to pregnancy with the patient, including gestational carrier and adoption.
Discussion of potential aortic and cardiovascular risks associated with pregnancy
In order to provide comprehensive preconception counseling, it is important to have recent imaging of the aorta to assess aortic root size with transthoracic echocardiogram and sometimes imaging of the entire vascular system with either computed tomography (CT) or magnetic resonance imaging (MRI) (20). The imaging results, along with the person’s medical history, are key factors in determining risks in pregnancy and risks for type A dissection in pregnancy, especially for individuals with Marfan syndrome (MFS), Loeys-Dietz syndrome (LDS), bicuspid aortic valve (BAV) and non-syndromic heritable thoracic aortic disease (nsHTAD). Aortic root dilation or aneurysm is a risk factor for dissection in genetic aortopathies. The recent American College of Cardiology/American Heart Association (ACC/AHA) guidelines on aortic disease state that the risk for AD related to a pregnancy is dependent on the genetic condition, the specific pathogenic variant, family history of AD and presence of rapid aortic root growth (≥3 mm/year) (20).
The aortic size threshold designating a person as higher risk for an aortic event in pregnancy is determined by genetic condition and pathogenic variant. For people with MFS, the risk for type A dissection is related to aortic root diameter; with <4.0 cm being lower risk (~1%) and higher risk at aortic diameter >4.5 cm (1,21-25). The risk for AD associated with pregnancy in LDS depends on the pathogenic variant; individuals with TGFBR1, TGFBR2, and SMAD3 are thought to be at higher risk for aortic events compared to individuals with TGFB2 and TGFB3 pathogenic variants (26,27). The risk of AD associated with pregnancy is higher in people with aortic size >4.0 cm in the higher risk pathogenic variants with LDS (28,29). With BAV and aortic dilation, the risk of AD increases at diameter >5 cm (30). With Turner syndrome, aortic size index (ASI) is used instead of aortic diameter and this is the aortic diameter divided by the body surface area as these individuals typically have shorter stature. An ASI >2.5 cm/m2 is considered high risk for AD associated with pregnancy and ASI 2–2.5 cm/m2 should proceed with pregnancy with caution as they may also be at higher risk (31-33). Additional risk factors for AD associated with pregnancy in individuals with Turner syndrome include structural heart disease, such as aortic coarctation and history of hypertension or hypertensive disorders of pregnancy (31-33). Individuals with nsHTAD have had AD associated with pregnancy and AD has occurred at smaller aortic diameters than guidelines from studies in MFS in some of these cases (34,35). With vascular Ehlers-Danlos syndrome (VEDS), AD is not predicted by aortic size and they can have vascular dissections in other arteries. Maternal mortality in pregnancy with VEDS ranges from 4–25% (36). The decision to proceed with pregnancy in VEDS is complex, and historically people with VEDS have been advised against pregnancy (20,37). However, individuals with null mutations may be at lower risk for complications in pregnancy (3).
Type B dissections related to pregnancy are more difficult to predict, occurring without descending thoracic aortic dilation, and do not appear to have identifiable risk factors (1,25,38). Type B dissection is responsible for 20–40% of AD in pregnancy in individuals with MFS (1,21,25,39).
In addition to risks for AD associated with pregnancy, it is important to discuss the potential risk of the event of a pregnancy expediting aortic root growth and the potential long-term effect on cardiovascular health. Although there have been some studies that showed no difference in aortic size after pregnancy, other studies have shown that the event of pregnancy can cause a small amount of growth in the aorta (40,41).
Considerations for prophylactic aortic repair prior to pregnancy
For individuals in the highest risk groups for AD based on their genetic condition and aortic root size (Table 2), prophylactic aortic root replacement can be considered to avoid a type A dissection during pregnancy (20). However, the decision to proceed with surgery for aortic root replacement is complex; shared decision-making and assessment of longitudinal aortic growth is important.
Table 2
| Genetic condition | Aortic root size (cm)/aortic size index (cm/m2) to consider prophylactic surgery |
|---|---|
| Marfan syndrome | >4.5 cm |
| 4.0–4.5 cm if there are other risk factors present (family history of aortic dissection, rapid aortic growth >3 mm/year) | |
| Loeys-Dietz syndrome | ≥4.0 cm if TGFBR1, TGFBR2, SMAD3 variants |
| ≥4.5 cm if TGFB2, TGFB3 variants | |
| Bicuspid aortic valve | ≥5.0 cm |
| Turner syndrome | ≥2.5 cm/m2 |
| Non-syndromic heritable thoracic aortic aneurysm disease | ≥4.5 cm |
Adapted from ACC/AHA guidelines (20). ACC/AHA, American College of Cardiology/American Heart Association.
If a person and their care team decide to proceed with prophylactic aortic root replacement surgery, valve-sparing is the preferred method; however, if the valve is replaced, a bioprosthetic valve is preferred. This is secondary to risks of maternal morbidity and mortality with mechanical valves in pregnancy and the risk to the fetus with oral anticoagulation therapy (42).
It is important to note that these individuals, especially those with MFS and LDS, are still at risk for ADs distal to the graft and may have a higher risk for type B dissection based on some smaller cohorts (43,44).
Determination of medication safety in pregnancy
The pre-conception visit is the ideal time to review a person’s medications as some medications prescribed to these persons should be avoided in pregnancy. The most common of these include angiotensin receptor blockers (ARBs), warfarin/coumadin, and certain types of beta-blockers (atenolol). Changing medications in preparation for pregnancy should be done under the expertise of their cardiologist and obstetric provider.
Discussion of contraception and alternatives to pregnancy
If an individual does not desire pregnancy, it is imperative to have an effective contraceptive plan in place. Additionally, patients should be counseled that if pregnancy is desired, it is best to have a pre-conception meeting and review risks and potential plans for a pregnancy. Some individuals may decide that based on the risks they do not want to carry a pregnancy, but they want to start a family. It is important to review alternatives to carrying a pregnancy including gestational carrier with in vitro fertilization, and adoption.
Discussion of heritability risks to offspring and options for genetic testing
The majority of genetic aortopathies are autosomal dominant, which purports a 50% chance of passing the condition on to each potential offspring. People with genetic aortopathies are strongly recommended to meet with a genetic counselor or geneticist prior to pregnancy to discuss these risks and the options for testing during pregnancy. To be able to perform genetic testing during pregnancy, a pathogenic variant must have been identified in one of the parents in order to identify that pathogenic variant in the offspring. Prenatal genetic testing can be performed at three points during the pregnancy: (I) testing an early embryo with pre-implantation genetic testing using in vitro fertilization; (II) testing in the first trimester of pregnancy with chorionic villus sampling; and (III) testing in the second trimester with amniocentesis (45,46). Ultimately, genetic testing during pregnancy is a personal choice and these decisions are based on the individual’s and family’s beliefs, morals, and values, but all options should be discussed with every patient.
Antepartum considerations
During pregnancy, it is important to have a multi-disciplinary team including maternal-fetal medicine, cardiology, anesthesia, and cardiovascular surgery, and to ensure that delivery will occur at a hospital with cardiothoracic surgery.
Aortic imaging surveillance should be performed each trimester with transthoracic echocardiogram, but individual factors including genetic condition, aortic root size, and rate of aortic growth may serve as indications for more frequent imaging (38,39). To assess the descending aorta, an MRI can be performed without gadolinium contrast as this is low risk to the fetus and is preferred over CT during pregnancy if it is not an emergency to avoid radiation.
For all individuals with genetic aortopathy, a beta-blocker is recommended during and after pregnancy to decrease shearing forces on the blood vessels during pregnancy (1,20,38,39,47). There are some studies that have shown an association of fetal growth restriction with beta-blocker use in pregnancy; however, metoprolol is the preferred medication during pregnancy and is less likely to have this effect than other beta-blockers (38,39,48). If the person has underlying hypertension, labetalol is the medication of choice (20).
Delivery considerations
The physiological changes during labor and delivery put further stress on the vasculature with a 30% increase in cardiac output with contractions during the first stage of labor, and the act of pushing with Valsalva increases cardiac output by as much as 50% (14). The physiological adaptations of the cardiovascular system in labor may put increased stress on the aorta. While there are relatively few reported cases of AD during delivery, the postpartum period is a high-risk time for dissection (1). The hormonal changes and delivery physiology may be partially responsible for the increase in risk postpartum (1,14,17,18). Expert opinion and guidelines about the method of delivery attempt to ensure controlled delivery for those at highest risk based on genetic condition and aortic root size. It is paramount to have a multi-disciplinary plan for delivery to achieve optimal maternal and fetal outcomes and to have contingency plans for an AD during pregnancy or the postpartum period (39,47).
Anesthesia considerations
The anesthesia team is a critical part of the cardio-obstetric team for patients at risk of AD. Adequate analgesia/anesthesia is recommended to temporize large fluctuations in blood pressure and heart rate. If labor and vaginal delivery are planned, regional anesthesia is a key part of the delivery plan (20).
The two methods for obstetric anesthesia include regional (spinal or epidural) and general endotracheal anesthesia. Neuraxial anesthesia causes blockade of the sympathetic nervous system which can lead to reflexive tachycardia and hypotension. A gradual epidural is preferred over a spinal due to the lesser degree of reflexive tachycardia and hypotension. Phenotypic characteristics of MFS, LDS, and other genetic aortopathies, such as dural ectasias, vascular anomalies, and scoliosis/kyphosis, may complicate the care plan for anesthesia (49,50). Dural ectasias or scoliosis with Herrington rods can be a challenge for regional anesthesia placement, but these conditions are not necessarily a contraindication. Secondary to these challenges, it is important for patients to have imaging of the spine preconception or an MRI of the spine during pregnancy. A predelivery consultation with the anesthesia team during the pregnancy is important to formulate a plan for anesthesia during the delivery (49,50).
General anesthesia is reserved for those who are not candidates for neuraxial anesthesia or when there is no time for neuraxial placement (such as in the case of some obstetric emergencies). General anesthesia in pregnancy has an increased risk of hypoventilation and inability to intubate due to the soft tissue changes of the oropharynx and upper airway. Additionally, general anesthesia is associated with increased aspiration risk and hemodynamic instability particularly during induction, which can place strain on the thoracic aorta. Therefore, general anesthesia should be reserved for true emergencies and neuraxial anesthesia should remain the standard of care for these patients.
Timing of delivery
There are no randomized trials to guide the timing of delivery among patients with genetic aortopathies. However, delivery at 39–39.6 weeks is recommended to decrease the risk of development of hypertensive disorders of pregnancy. For patients with higher-risk conditions, including those with VEDS, those with dilated aortic root in the high-risk range, or chronic dissection, delivery is indicated in the late preterm-early term time period (34 to 37 weeks). Ultimately, the timing of delivery should be a multi-disciplinary discussion in shared decision-making with the patient and their family, weighing the maternal risks associated with their disease and the risks of prematurity to the neonate.
Mode of delivery
There are no randomized controlled trials to determine the safest delivery method for individuals with genetic aortopathy. Vaginal delivery physiologically has less rapid fluid shifts, decreased pain, and a shorter recovery period for patients. In individuals where the aorta is not significantly dilated, vaginal delivery is often performed with methods to lessen hemodynamic stress including regional anesthesia and an expedited second stage of labor with assisted vaginal delivery to avoid prolonged Valsalva (20,39,47). In patients with MFS and aortic root <4.5 cm, vaginal delivery with regional anesthesia and operative vaginal delivery are the preferred methods for delivery (4,47).
Cesarean delivery is recommended for individuals in the higher-risk category, although there is no clear evidence that labor increases risks for AD and cesarean delivery has not been shown to be protective (20,38). In patients with aortic root or ascending greater than 4.5 cm, rapid aortic root growth, or chronic AD, cesarean delivery is recommended (20).
Postpartum considerations
After delivery, the risk for AD associated with pregnancy still exists and should be monitored until 6–12 weeks postpartum. Beta-blocker therapy should be continued until 6–12 weeks postpartum and imaging after delivery is recommended. The timing and method of imaging depends on the concern for AD. In those at highest risk, imaging may be done prior to discharge from the hospital and then repeated at 3–6 months postpartum (20,38,39).
Mouse models of MFS and VEDS show that breastfeeding and oxytocin, important for milk let down, may increase the risk of postpartum dissection (51,52). However, these findings have not yet been replicated in human studies. Physicians should engage patients in nuanced discussions of potential risks and benefits with the patient and support persons, ensuring full consideration of the maternal and neonatal benefits of breastfeeding. Ultimately, the patient should make their own decisions about whether to breastfeed.
Acute aortic event during pregnancy
An acute AD during pregnancy is a cardiovascular and obstetric emergency. It is important to prepare the entire team with the contingency plan of care if an AD is suspected, as rapid coordination of obstetrics, cardiology, radiology, anesthesia and cardiovascular surgery is required to treat the maternal-fetal dyad in an expedited manner.
CT is the fastest and preferred method for diagnosis if there is a high index of suspicion for AD based on symptoms and vital signs (20,53). The American College of Obstetrics and Gynecology (ACOG) states that ionizing radiation used in imaging should not be withheld from a pregnant person if it is thought to be necessary. Additionally, the ionizing radiation from one CT scan is not harmful to the fetus (54).
The treatment for AD in pregnancy is dependent on the type of AD and the gestational age of the fetus (20,55). In patients with acute type A dissection, surgery with aortic repair is necessary. However, the decision to perform surgery leaving the fetus in utero versus cesarean delivery prior to aortic surgery depends on the gestational age of the fetus (Figure 2). There is no defined gestational age where delivery is recommended prior to surgery, but the recent ACC/AHA guidelines set this threshold at 26 weeks (20). Ideally, this should be a shared decision between the patient and their cardio-obstetric care team weighing the risks of prematurity versus the risks to the fetus during cardiopulmonary bypass (CPB).
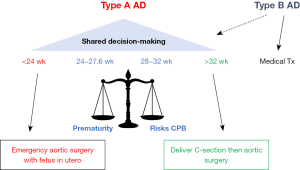
Fetal morbidity and mortality are high with CPB with fetal death estimated at 25–40% (56). Pulsatile flow is recommended to minimize risks of hypoxia to the fetus. Pulsatile blood flow can also increase total blood flow by lowering systemic vascular resistance, which can prevent progressive hypoxia to the fetal-placental unit during surgery. Normothermic transfusion is preferred over hypothermic to avoid fetal bradycardia. Flow rates of CPB >2.4 L/min to preserve placental perfusion and high arterial pressure mean arterial pressure (MAP) 70–75 are also recommended (56-59).
In persons experiencing an acute type B dissection, medical therapy is recommended unless endovascular or surgical treatment is thought to be necessary (20,55).
Special clinical scenarios with aortic disease in pregnancy
Chronic AD in pregnancy
There is limited information on pregnancy-related aortic risks with chronic dissection in pregnancy. There is theoretical concern that the physiological cardiovascular and hormonal changes of pregnancy will destabilize a chronic dissection, and pregnancy is therefore classified as high risk (20). A planned cesarean delivery is recommended to have a controlled delivery although cesarean delivery has not been shown to be protective.
Conclusions
AD associated with pregnancy is rare but has high stakes for the patient and their pregnancy. It is most likely to occur in the third trimester or postpartum period. In individuals with genetic aortopathy, management guidelines exist to optimize a favorable maternal and fetal outcome. It is important to have a multi-disciplinary team and capabilities for cardiovascular surgery to give the best care to these individuals. The decision to pursue a pregnancy is ultimately at the discretion of the individual, and it is the duty of the cardio-obstetric team to ensure that they understand the risks of pregnancy and the plan of care.
Acknowledgments
We would like to thank Andrew Creamer, Open Science Librarian, Brown University, Warren Alpert Medical School for assisting with the literature search for this article.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Braverman AC, Mittauer E, Harris KM, et al. Clinical Features and Outcomes of Pregnancy-Related Acute Aortic Dissection. JAMA Cardiol 2021;6:58-66. [Crossref] [PubMed]
- Smok DA. Aortopathy in pregnancy. Semin Perinatol 2014;38:295-303. [Crossref] [PubMed]
- Cottrell J, Calhoun J, Szczepanski J, et al. Aortic root valve-sparing repair and dissections in Marfans syndrome during pregnancy: A case series. J Card Surg 2020;35:1439-43. [Crossref] [PubMed]
- Narula N, Devereux RB, Malonga GP, et al. Pregnancy-Related Aortic Complications in Women With Marfan Syndrome. J Am Coll Cardiol 2021;78:870-9. [Crossref] [PubMed]
- Warner D, Holmes KW, Afifi R, et al. Emergency vascular surgical care in populations with unique physiologic characteristics: Pediatric, pregnant, and frail populations. Semin Vasc Surg 2023;36:340-54. [Crossref] [PubMed]
- Sawlani N, Shroff A, Vidovich MI. Aortic dissection and mortality associated with pregnancy in the United States. J Am Coll Cardiol 2015;65:1600-1. [Crossref] [PubMed]
-
Maternal Mortality Rates in the United States 2021 . Available online: https://www.cdc.gov/ - Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003;76:309-14. [Crossref] [PubMed]
- De Martino A, Morganti R, Falcetta G, et al. Acute aortic dissection and pregnancy: Review and meta-analysis of incidence, presentation, and pathologic substrates. J Card Surg 2019;34:1591-7. [Crossref] [PubMed]
- Jalnapurkar S, Shmueli H, Goland S, et al. Pregnancy associated aortic complications requiring surgery: a review of contemporary experience in 150 cases between 2000 to 2020. J Am Coll Cardiol 2021;77:3398.
- Rommens KL, Sandhu HK, Miller CC 3rd, et al. In-hospital outcomes and long-term survival of women of childbearing age with aortic dissection. J Vasc Surg 2021;74:1135-1142.e1. [Crossref] [PubMed]
- Kamel H, Roman MJ, Pitcher A, et al. Pregnancy and the Risk of Aortic Dissection or Rupture: A Cohort-Crossover Analysis. Circulation 2016;134:527-33. [Crossref] [PubMed]
- Nasiell J, Lindqvist PG. Aortic dissection in pregnancy: the incidence of a life-threatening disease. Eur J Obstet Gynecol Reprod Biol 2010;149:120-1. [Crossref] [PubMed]
- Clark SL, Cotton DB, Lee W, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol 1989;161:1439-42. [Crossref] [PubMed]
- Robson SC, Hunter S, Boys RJ, et al. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989;256:H1060-5. [Crossref] [PubMed]
- Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin 2012;30:317-29. [Crossref] [PubMed]
- Campisi D, Bivona A, Paterna S, et al. Oestrogen binding sites in fresh human aortic tissue. Int J Tissue React 1987;9:393-8.
- Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol 1967;83:336-41.
- Curtis SL, Swan L. Aortopathy in pregnancy. Heart 2022;108:1851-7. [Crossref] [PubMed]
- Writing Committee Members. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;80:e223-393. [Crossref] [PubMed]
- Pyeritz RE. Maternal and fetal complications of pregnancy in the Marfan syndrome. Am J Med 1981;71:784-90. [Crossref] [PubMed]
- Rossiter JP, Repke JT, Morales AJ, et al. A prospective longitudinal evaluation of pregnancy in the Marfan syndrome. Am J Obstet Gynecol 1995;173:1599-606. [Crossref] [PubMed]
- Lind J, Wallenburg HC. The Marfan syndrome and pregnancy: a retrospective study in a Dutch population. Eur J Obstet Gynecol Reprod Biol 2001;98:28-35. [Crossref] [PubMed]
- Uchida T, Ogino H, Ando M, et al. Aortic dissection in pregnant women with the Marfan syndrome. Kyobu Geka 2002;55:693-6.
- Roman MJ, Pugh NL, Hendershot TP, et al. Aortic Complications Associated With Pregnancy in Marfan Syndrome: The NHLBI National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC). J Am Heart Assoc 2016;5:e004052. [Crossref] [PubMed]
- Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet 2012;44:916-21. [Crossref] [PubMed]
- Marsili L, Overwater E, Hanna N, et al. Phenotypic spectrum of TGFB3 disease-causing variants in a Dutch-French cohort and first report of a homozygous patient. Clin Genet 2020;97:723-30. [Crossref] [PubMed]
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788-98. [Crossref] [PubMed]
- Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: Results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet 2016;9:548-58. [Crossref] [PubMed]
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic Dissection in Patients With Bicuspid Aortic Valve-Associated Aneurysms. Ann Thorac Surg 2015;100:1666-73; discussion 1673-4. [Crossref] [PubMed]
- Hynes JS, Kuller JA, Goldstein SA, et al. Increased Risk of Aortic Dissection Associated With Pregnancy in Women With Turner Syndrome: A Systematic Review. Obstet Gynecol Surv 2020;75:566-75. [Crossref] [PubMed]
- Donadille B, Bernard V, Christin-Maitre S. How can we make pregnancy safe for women with Turner syndrome? Am J Med Genet C Semin Med Genet 2019;181:100-7. [Crossref] [PubMed]
- Silberbach M, Roos-Hesselink JW, Andersen NH, et al. Cardiovascular Health in Turner Syndrome: A Scientific Statement From the American Heart Association. Circ Genom Precis Med 2018;11:e000048. [Crossref] [PubMed]
- Regalado ES, Guo DC, Estrera AL, et al. Acute aortic dissections with pregnancy in women with ACTA2 mutations. Am J Med Genet A 2014;164A:106-12. [Crossref] [PubMed]
- Wallace SE, Regalado ES, Gong L, et al. MYLK pathogenic variants aortic disease presentation, pregnancy risk, and characterization of pathogenic missense variants. Genet Med 2019;21:144-51. [Crossref] [PubMed]
- Murray ML, Pepin M, Peterson S, et al. Pregnancy-related deaths and complications in women with vascular Ehlers-Danlos syndrome. Genet Med 2014;16:874-80. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Cauldwell M, Steer PJ, Curtis SL, et al. Maternal and fetal outcomes in pregnancies complicated by Marfan syndrome. Heart 2019;105:1725-31. [Crossref] [PubMed]
- Campens L, Baris L, Scott NS, et al. Pregnancy outcome in thoracic aortic disease data from the Registry Of Pregnancy And Cardiac disease. Heart 2021;107:1704-9. [Crossref] [PubMed]
- Donnelly RT, Pinto NM, Kocolas I, et al. The immediate and long-term impact of pregnancy on aortic growth rate and mortality in women with Marfan syndrome. J Am Coll Cardiol 2012;60:224-9. [Crossref] [PubMed]
- Renard M, Muiño-Mosquera L, Manalo EC, et al. Sex, pregnancy and aortic disease in Marfan syndrome. PLoS One 2017;12:e0181166. [Crossref] [PubMed]
- Ng AP, Verma A, Sanaiha Y, et al. Maternal and Fetal Outcomes in Pregnant Patients With Mechanical and Bioprosthetic Heart Valves. J Am Heart Assoc 2023;12:e028653. [Crossref] [PubMed]
- Braverman AC, Moon MR, Geraghty P, et al. Pregnancy after aortic root replacement in Loeys-Dietz syndrome: High risk of aortic dissection. Am J Med Genet A 2016;170:2177-80. [Crossref] [PubMed]
- Williams D, Lindley KJ, Russo M, et al. Pregnancy after Aortic Root Replacement in Marfan's Syndrome: A Case Series and Review of the Literature. AJP Rep 2018;8:e234-40. [Crossref] [PubMed]
- Preimplantation Genetic Testing. ACOG Committee Opinion Summary, Number 799. Obstet Gynecol 2020;135:752-3. [Crossref] [PubMed]
- Dukhovny S, Norton ME. What are the goals of prenatal genetic testing? Semin Perinatol 2018;42:270-4. [Crossref] [PubMed]
- Minsart AF, Mongeon FP, Laberge AM, et al. Obstetric and cardiac outcomes in women with Marfan syndrome and an aortic root diameter ≤ 45mm. Eur J Obstet Gynecol Reprod Biol 2018;230:68-72. [Crossref] [PubMed]
- Duan L, Ng A, Chen W, et al. Beta-blocker subtypes and risk of low birth weight in newborns. J Clin Hypertens (Greenwich) 2018;20:1603-9. [Crossref] [PubMed]
- Weinstein J, Shinfeld A, Simchen M, et al. Anesthesia in Parturients Presenting with Marfan Syndrome. Isr Med Assoc J 2021;23:437-40.
- Cronin J, Bazick Cuschieri H, Dong X, et al. Anesthesia considerations for cesarean delivery in a patient with Loeys-Dietz syndrome. A A Case Rep 2015;4:47-8. [Crossref] [PubMed]
- Habashi JP, MacFarlane EG, Bagirzadeh R, et al. Oxytocin antagonism prevents pregnancy-associated aortic dissection in a mouse model of Marfan syndrome. Sci Transl Med 2019;11:eaat4822. [Crossref] [PubMed]
- Bowen CJ, Calderón Giadrosic JF, Burger Z, et al. Targetable cellular signaling events mediate vascular pathology in vascular Ehlers-Danlos syndrome. J Clin Invest 2020;130:686-98. [Crossref] [PubMed]
- Rimmer L, Heyward-Chaplin J, South M, et al. Acute aortic dissection during pregnancy: Trials and tribulations. J Card Surg 2021;36:1799-805. [Crossref] [PubMed]
- Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol 2017;130:e210-6. Erratum in: Obstet Gynecol 2018;132:786. [Crossref] [PubMed]
- Ma WG, Zhu JM, Chen Y, et al. Aortic dissection during pregnancy and postpartum in patients with Marfan syndrome: a 21-year clinical experience in 30 patients. Eur J Cardiothorac Surg 2020;58:294-301. [Crossref] [PubMed]
- Jha N, Jha AK, Chand Chauhan R, et al. Maternal and Fetal Outcome After Cardiac Operations During Pregnancy: A Meta-Analysis. Ann Thorac Surg 2018;106:618-26. [Crossref] [PubMed]
- Champsaur G, Parisot P, Martinot S, et al. Pulsatility improves hemodynamics during fetal bypass. Experimental comparative study of pulsatile versus steady flow. Circulation 1994;90:II47-50.
- Barth WH Jr. Cardiac surgery in pregnancy. Clin Obstet Gynecol 2009;52:630-46. [Crossref] [PubMed]
- Sarkar MS, Desai P. Pregnancy and cardiac surgery. In: Sarkar SM, Gvalani S. Cardiac Anesthesia: Practical Aspects. JP Medical Ltd.; 2015:233-8.