Hybrid atrial fibrillation ablation
Introduction
Pathophysiology of AF
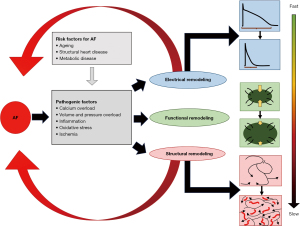
Atrial fibrillation (AF) is the most common sustained arrhythmia. It often starts with short paroxysms and then becomes progressively sustained over time. This phenomenon is the consequence of three important forms of remodeling: electrical, functional and structural remodeling (see Figure 1) (1). During electrical remodeling, rapid rates of AF lead to shortening of action potential duration and atrial effective refractory period, facilitating rapid conduction and re-entrant wavelets to meander and reactivate excitable tissue. Functional remodeling is due to the degradation of contractile proteins. This atrial dysfunction leads to low blood flow velocity and thereby significantly contributes to the thrombo-embolic risk associated with AF. Both electrical and functional remodeling occur fast and are reversible to a certain extent. Structural remodeling is due to irreversible changes in the architecture of the atrial wall and underlies the stabilization and perpetuation of the fibrillating process. Important determinants of structural remodeling are the development of endomysial fibrosis in between muscle bundles and epicardial adipose tissue through fatty infiltration within the atrial wall or via secretion of paracrine modulators of myocardial inflammation and oxidative stress (2,3). Pathogenic factors and risk factors for AF act as catalysts in the process of electrical, contractile and structural remodeling (Figure 1).
AF mechanisms
In general, AF requires a trigger to initiate and a substrate to maintain the arrhythmia. Over time, AF is believed to progress from a ‘hierarchical’ organization, where one trigger is initiating paroxysms of the arrhythmia, to an ‘anarchical’ organization, where the arrhythmia persists as re-entrant fibrillating waves perpetuated by the underlying AF substrate (Figure 2) (4).
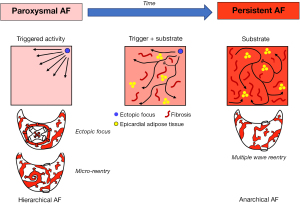
The most well documented source of triggers are the myocardial sleeves of the pulmonary veins (PVs) (5), but triggers can also originate from the ligament of Marshall (LOM), the superior caval vein (SCV), the left atrial appendage (LAA) (6) and other non-specified regions of the atria (1). The atrial substrate develops due to structural remodeling by increasing the atrial mass and surface, thereby promoting the perpetuation of fibrillating waves. Important mechanisms that enhance the persistence of AF are longitudinal and endo-epicardial dissociation of electrical activity (4), transmural conduction (7) and bundle rearrangement following atrial dilatation (8). The left atrial (LA) posterior wall (PW) is an interesting region in that sense, as its thin wall consists of overlapping muscle layers with different orientation (9). Such acute transitions in fiber architecture may promote conduction block and re-entry, especially because persistence of AF goes hand in hand with structural remodeling including bundle rearrangement and development of endomysial tissue fibrosis. This suggests that the PW is an important region for the development of an AF substrate.
Modern rhythm control therapy uses minimally invasive interventions, including both catheter and surgical ablation, to target the triggers as well as to modify the substrate responsible for the initiation and perpetuation of AF.
Approaches to AF ablation
Catheter based AF ablation techniques
Since the seminal paper of Haïssaguerre, in which the PVs were identified as common triggers of ectopic foci leading to paroxysms of AF, catheter-based techniques were developed to attempt electrical isolation of the PVs (5). Catheter-based PV isolation (PVI) is an effective strategy with reported success rates of 80% in paroxysmal AF (pAF) patients (10). However, in longstanding, persistent AF (persAF) patients, arrhythmia free survival after PVI is suboptimal. First of all, it is assumed that PVI alone is insufficient for preventing AF recurrences in patients with more persistent forms of AF due to the need for more extensive ablation of non-PV triggers, such as the LA PW, the LAA or the LOM (11). Secondly, establishing durable long-lasting transmural lesions to prevent reconduction of the PVs and of linear lesions is one of the major weaknesses of catheter ablation (CA) and incomplete lesions may even promote atrial tachyarrhythmias (ATA) (12). In the STAR-AF II trial, single-procedural efficacy without the use of antiarrhythmic drugs (AAD) was only 41% at 18 months of follow-up for PVI alone and outcomes did not improve with additional ablation of complex fractionated atrial electrograms or linear lesions (13). Likewise, the more recently published CAPLA trial also demonstrated poor efficacy outcomes of catheter ablation (CA) in persAF patients, and randomization of PVI in combination with PW isolation (PWI) versus PVI alone as a first-time CA did not improve freedom of ATA at 12 months (52.4% vs. 53.6% respectively, P=0.98) (14). The clinical implication is important as these results teach us that empirical PWI as part of an endocardial CA strategy for persAF is not beneficial with regard to rhythm outcome. Furthermore, the addition of alcohol ablation of the LOM or empirical LAA isolation to endocardial PVI only results in a modest improvement of success rates (15,16).
Pulsed field ablation (PFA) is a relatively new and promising technique that applies ultrarapid electrical fields to the tissue to induce homogeneous lesions. Although the technique seems efficacious and safe in pAF patients (17), results are still preliminary and outcomes in more persistent forms of AF require further investigation before PFA may be implemented in a hybrid approach.
Towards a minimally invasive thoracoscopic AF ablation
In 1987, Dr. Cox performed the first Cox-Maze procedure (18). Based on animal models, the procedure involved cutting and sewing of both atria to interrupt macro re-entrant circuits that were thought to drive AF. Although the procedure was effective in restoring sinus rhythm (SR), it was not widely adopted by surgeons due to its technical complexity and risk of pacemaker implantation.
Although the Cox-Maze procedure is very effective in creating transmural lesions, and its lesion set is still the golden-standard for AF ablation for many surgeons, it requires the use of cardiopulmonary bypass. As such, the quest for a surgical technique that is as efficacious as the original Cox-Maze procedure, but less invasive, has led to the development of minimally invasive (key-hole) surgical approaches. Hence, a standalone minimally invasive, bilateral video assisted thoracoscopic procedure on the beating heart was developed. Its superiority in rhythm outcome compared to CA (19) is mainly attributable to the fact that it is a surgical procedure with direct vision of the anatomy, uses bipolar biparietal radiofrequency (RF) clamps and can isolate epicardial accessible non-PV sources that are responsible for ectopic activity and can serve as a substrate that can initiate and perpetuate the arrhythmia, such as the LOM or ganglionated plexi, as well as being able to mechanically and electrically exclude the LAA.
Although results of such a thoracoscopic approach are good, an important shortcoming of the technique is that the surgeon is in fact blind to the underlying electrophysiological properties of the atria. Moreover, some lesions cannot be treated solely from the epicardium, such as a mitral or cavo-tricuspid isthmus line. Finally, epicardial testing of entrance and exit block may not always be as reliable as endocardial electrophysiological validation.
Hybrid AF ablation
In order to overcome their mutual shortcomings and to combine the strengths of a CA and a thoracoscopic approach, our group introduced the hybrid AF approach in the Maastricht University Medical Center (MUMC) in 2010 (20). Given the incomplete understanding of underlying AF mechanisms and the complexity of persistent forms of AF, the concept of combining the strengths of a minimally invasive epicardial with a percutaneous endocardial approach was originated (Figure 3). With a hybrid procedure, the endocardial approach can be performed single-staged or two-staged, e.g., within six months after the epicardial ablation. It is important to note that the concept of the hybrid procedure requires both an epicardial and an endocardial approach. As such, an endocardial procedure only in case of failure after thoracoscopic AF ablation is in fact not a hybrid but a redo procedure. The strength of a hybrid procedure is highlighted by its complementary nature: the surgeon has direct three-dimensional visualization of the anatomy and can create long-lasting epicardial lesions while the electrophysiologist (EP) uses high-resolution endocardial maps to visualize the underlying substrate. As such, the close cooperation between the surgeon and the EP allows tailoring of the lesion set in the same procedure (a nice example is depicted in Figure 4) (21). This is of course especially true for the single-staged procedure and less for the two-staged procedure. In both cases, effective epicardial ablation is combined with endocardial electrophysiological confirmation. At the same time, regular ATAs that arise during the single-staged procedure can be addressed and touch-up of residual epicardial conduction gaps can be performed to achieve bidirectional block. Furthermore, additional electrophysiological targets can be identified to minimize recurrent ATA.
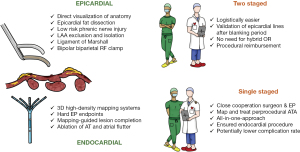
Technical aspects of hybrid AF ablation
Surgical approaches in hybrid AF ablation
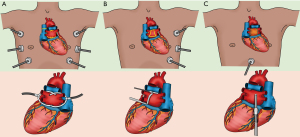
In general, the goal of the surgical part of hybrid AF ablation is to isolate the PVs and the LOM, create a box lesion and exclude the LAA (22). This can be performed via a thoracoscopic (both bilateral and unilateral) or a subxiphoid approach (Figure 5). The latter does not allow for complete epicardial PV and LOM isolation and requires additional thoracoscopic access for LAA management.
Energy sources
To date, all approaches use RF energy that can be applied uniparietal (at one side of the atrial wall) or biparietal (at both sides of the atrial wall) using a unipolar or bipolar modality. The use of biparietal bipolar RF clamps is considered superior in creating transmural and durable lesions compared to uniparietal bipolar and unipolar RF devices (23,24).
Besides the differences in polarity, available devices also use different types of RF energy, irrigated and non-irrigated. The mechanism of tissue injury in response to RF energy is thermal, and cooling is crucial to create lesions in a safe fashion (25). Using irrigated (wet) energy ensures a more controlled way of heating tissue by continuously adjusting its power output to tissue impedance and cooling the tissue surface by adding saline, whereas non-irrigated (dry) RF energy sources only heat affected tissue. Therefore, the use of non-irrigated RF ablation devices can overcome the possible side effects of over-heating, such as microbubbles, tissue coagulum and charring (26).
Bilateral epicardial techniques
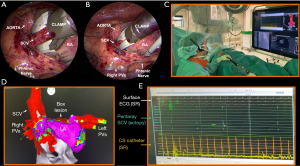
Bilateral thoracoscopic epicardial ablation techniques are performed in supine position (Figure 5). The first technique uses a single clamping device with irrigated bipolar RF energy (Medtronic Cardioblate Gemini, Medtronic, MN, USA). The two glides are placed in the transverse and oblique sinus to guide the clamp and overlapping lesions are performed from both sides thereby isolating the PVs and the PW in one continuous lesion (Figure 5A). The second technique uses dry RF energy via left and right-shaped bipolar clamps to isolate the PVs and an uniparietal bipolar linear RF device to connect both superior and inferior PVs (Isolator Clamp and Coolrail, AtriCure, OH, USA) to complete the ‘box’. More recently, a bilateral technique to isolate the PVs and the PW with bipolar biparietal clamps (Isolator Clamp, AtriCure, OH, USA) using dry energy has been introduced (ABC), but its efficacy remains to be determined. An advantage of the bilateral approaches is that the SCV can also be addressed (Figure 4).
Unilateral techniques
Both left- and right-sided unilateral thoracoscopic approaches using a bipolar RF clamp and a linear ablation device (Isolator and Coolrail, AtriCure, OH, USA) have been introduced (27,28). The potential advantages of a left-sided technique (Figure 5B) are tissue dissection away instead of towards the heart, PVI with the convex side of the bipolar clamp towards the heart, a larger lung capacity during single right lung ventilation and direct visualization during LAA exclusion, whilst a right-sided technique allows for SCV isolation. Be that as it may, any unilateral technique has the potential advantage to minimize surgical trauma, reduce postoperative pain and prevent complications at the contralateral side (22). More recently, an all bipolar RF clamp technique from the right side has been introduced, but the efficacy remains to be determined and it requires the use of both irrigated and non-irrigated RF clamps, thereby increasing costs significantly (29).
Subxiphoid technique
To minimize surgical invasiveness and complexity even further, the “Convergent” procedure was introduced (30). Instead of approaching the heart via the thorax, this technique is characterized by a subxiphoid (or transdiaphragmatic) access (Figure 5C). Epicardial ablation of the LA PW is performed by creating several parallel lesions in between the left and right PVs, using a vacuum assisted, unipolar RF ablation device (EPi-sense, AtriCure, OH, USA). Although promising, this subxiphoid approach does not allow LAA exclusion and the access to the LA is limited by the pericardial reflections. As a result, complete PWI is challenging and complementary endocardial ablation of the PVs and PW is needed.
LAA exclusion techniques
Thoracoscopic LAA occlusion on the beating heart can be challenging and can be performed using a clipping device (AtriClip, AtriCure, OH, USA), a stapler device or a snaring device (Lariat, AtriCure, OH, USA). Most safety and efficacy data are reported with the use of the AtriClip (31) which is also the most widely used device.
Electrophysiological validation
Confirmation of conduction block over the ablation lesions is of uttermost importance, as incomplete lines can lead to highly symptomatic ATAs. Although epicardial EP validation allows for fast validation with direct vision of the anatomy and without necessitating an EP working station, it also has important pitfalls. False-positive confirmation of conduction block can occur because information on the underlying substrate is lacking. For example, accidental epicardial testing of exit and entrance block on a fibrotic strand located in the box or inadequate contact pacing may lead to a false confirmation of box isolation (22). Secondly, epicardial confirmation of a non-isolated LA PW requires re-ablation of all epicardial lines. As the conduction gap following an extensive epicardial lesion set is most often located at the endocardium, endocardial EP mapping enables exact localization of the conduction gap and consequent delivery of a few targeted endocardial ablation points to complete the lesion set can be performed. Interestingly, Vroomen et al. (32) compared the correspondence of epicardial with endocardial EP validation during hybrid AF ablation. Although in general both validation techniques corresponded well, bidirectional block of the right superior vein (RSPV) was not confirmed from the endocardium in 15% of all patients, which highlights the importance of the EP. The latter was also demonstrated in the HARTCAP-AF trial (33), where after epicardial ablation eight patients (42%) required endocardial touch-up ablation due to epicardial conduction gaps.
Efficacy and safety of hybrid AF ablation in meta-analyses
Efficacy
Over the past years, several meta-analyses have discussed rhythm and safety outcomes following hybrid AF ablation (34-40) (Table 1). Based on these studies, the overall success rate of a hybrid AF procedure, in terms of freedom from ATA recurrences until twelve months post-procedure off AAD, varies between 59–88%. Due to the scarcity of available randomized controlled trials (RCTs), these efficacy outcomes were mostly based on single arm studies (either an observational or trial design) of patients undergoing hybrid AF ablation and in some studies compared to surgical epicardial AF ablation alone (37) or percutaneous endocardial AF ablation (34). Importantly, marked statistical and clinical heterogeneity between studies was present. Concerning statistical heterogeneity, the scarcity of available RCTs directly comparing hybrid ablation with surgical or catheter AF ablation resulted in pooled efficacy outcomes derived from observational studies, single-arm studies, or propensity matched studies in a random-effects model. Per definition, effect estimates based on single-arm studies in a random effects model come with a high degree of inter-study variance and statistical heterogeneity.
Table 1
| First author | Year | Success rate allowing AAD (%) |
Success rate off AAD (%) |
Approach |
|---|---|---|---|---|
| Baudo (34) | 2023 | 74±3 | Thoracoscopic + trans-diaphragmatic | |
| Bisleri (35) | 2023 | 75 [69–81] | Thoracoscopic + subxiphoidal + trans-diaphragmatic | |
| van der Heijden (40) | 2019 | 71 [61–80] | Thoracoscopic + subxiphoid | |
| Jiang (36) | 2018 | 73 [64–81] | Thoracoscopic + trans-diaphragmatic (laparoscopic) | |
| Pearman (37) | 2013 | 63 [52–75] | Thoracoscopic + trans-diaphragmatic | |
| Syed (38) | 2015 | A. 88 [133–151] | A. Thoracoscopic bilateral | |
| B. 73 [47–64] | B. Right-sided thoracoscopic | |||
| C. 59 [80–135] | C. Subxiphoid | |||
| Je (39) | 2015 | 71* | Thoracoscopic + subxiphoid |
Data were presented as mean ± SD or [95% CI]. *, no CI or SD given. AAD, antiarrhythmic drugs; CI, confidence interval; SD, standard deviation.
Perhaps one of the most important factors responsible for the clinical heterogeneity between published studies, explaining the wide variety in efficacy outcomes, is that all meta-analyses included at least one study reporting on hybrid ablation via a subxiphoid and/or trans-diaphragmatic approach. Subsequently, the efficacy and safety outcomes of this approach were pooled with those after a thoracoscopic hybrid AF procedure. Although in theory the lesion set is comparable, the technique is quite different. In most meta-analyses, the authors reported that efficacy results following a subxiphoid or trans-diaphragmatic seemed inferior compared to rhythm outcomes via a thoracoscopic access. This can be explained by several factors. First, while thoracoscopic AF ablation allows for biparietal bipolar RF isolation of the PVs, or even complete PWI when using the Gemini-S ablation system, the subxiphoid and trans-diaphragmatic approaches require a unipolar vacuum assisted RF device for the epicardial PWI, and endocardial PVI is required to complete the lesion set. As demonstrated in the CONVERGE trial (30), where persAF patients were randomized between subxiphoid hybrid ablation and CA, patients in the hybrid arm showed a significant higher freedom from AF recurrences than the catheter arm (53.5% vs. 32.0% respectively, P=0.013). Interestingly, these efficacy outcomes following subxiphoid hybrid ablation are low compared to a thoracoscopic hybrid approach. Also Pearman et al. (37) described inferior efficacy outcomes at twelve months for trans-diaphragmatic access compared to thoracoscopic access (OR 0.72; 95% CI: 0.61–0.8; P=0.001). As described previously, the use of a biparietal bipolar RF clamp is associated with a higher degree of transmurality and consequently improves rhythm outcomes compared to unipolar RF energy (24). Moreover, thoracoscopic hybrid AF ablation has the major advantage of simultaneously managing the LAA as well. Several studies in the abovementioned meta-analyses have reported greater efficacy outcomes in terms of rhythm outcome when the LAA was surgically excluded (37,40), either by clipping or stapling. In our opinion, LAA management is of paramount importance in AF management. Not only does surgical epicardial exclusion of the LAA significantly lower the risk of ischemic stroke or systemic embolism, as described in the LAAOS-III trial (41), but empirical electrical isolation of ectopic foci originating from the LAA and mass reduction also improves long-term freedom from ATAs, as described in the BELIEF trial (16). Overall, the efficacy outcomes following hybrid AF ablation of currently presented meta-analyses may be obscured due to the inclusion of studies that reported on a subxiphoid approach and did not target the LAA.
Complications
Aside from implementing a technique that is effective, patient safety is of top priority. Although minimally invasive, surgical AF ablation naturally carries a greater risk for complications than a truly minimally invasive percutaneous catheter approach, overall complications were low in the abovementioned meta-analyses that reported on safety outcomes after hybrid AF ablation. Again, clinically relevant differences may be present between studies that reported on different techniques. For example, Pearman et al. reported higher rates of major complications for a trans-diaphragmatic access compared to thoracoscopic (OR 1.05; 95% CI: 1.00–1.11; P=0.04). Furthermore, the meta-analysis by Mhanna et al. (42) indicated a trend towards a better safety profile for thoracoscopic access, although frequentist statistics did not show a significant difference between subgroups based on the P value, potentially due to the limited sample size.
When comparing safety outcomes with CA, Edgerton et al. (43) found that a subxiphoid approach led to more procedural complications. In the meta-analysis by van der Heijden et al. (40), overall complications following mainly thoracoscopic hybrid and percutaneous catheter AF ablation were both low, but hybrid ablation still resulted in slightly more complications. In addition, it is often speculated that due to the nature of a hybrid procedure, by implementing two procedures in one, the individual risk of complications of each procedure can be accumulated to define the risk of one hybrid AF procedure. Of course, both a surgical and catheter procedure carry specific risks, such as a thoracic wound infection or a groin hematoma respectively. Nonetheless, this does not always mean that one can simply add both risks up to define the total complication risk for a hybrid AF procedure. First, certain complications may only occur once, such as a pneumonia during hospitalization, and cannot be doubled by adding the endocardial validation to the surgical procedure. Secondly, some of the risks of a percutaneous approach are minimized or even eliminated when first preceded by a surgical ablation. For example, phrenic nerve injury is an important complication after stand-alone CA, but this risk becomes limited during hybrid ablation as the pericardium has already been opened during the epicardial procedure. Likewise, the risk of PV stenosis by extensive ablation around the PVs during stand-alone CA is reduced during hybrid ablation, because in most cases only a simple endocardial touch-up (or no touch-up at all) is required to obtain complete PVI due to the ability of creating transmural lesions during the epicardial part of the procedure. Finally, in the rare case of bleeding or perforation during the catheter part of the procedure, the incidence of a cardiac tamponade as part of a hybrid procedure is very unlikely as the pericardium has previously been opened.
Efficacy and safety of thoracoscopic- and hybrid AF ablation in randomized data
Thoracoscopic versus catheter ablation
Several RCTs compared efficacy and safety rates following a totally thoracoscopic surgical AF ablation compared to CA (Table 2). In the FAST trial, 129 pAF and persAF patients were randomized to thoracoscopic AF ablation using bipolar RF or unipolar RF CA (19). Compared to CA, thoracoscopic ablation was more effective in terms of freedom from ATA recurrences until twelve months of follow-up (66% vs. 37%, P=0.002), but also resulted in more complications (34% vs. 16%, P=0.027). Even after long-term follow-up of seven years, thoracoscopic AF ablation was still superior with only 56% of all patients having documented ATA recurrences compared to 87% in the catheter group (P<0.001) (48). The high complication rate (34%) stands in contrast with currently reported safety outcomes. This can be explained by the fact that the complications in the FAST trial tended to result mostly from mechanical injury during the procedure. This trial was performed in 2007–2010 and since then, techniques and tools have been improved and especially the experience of operators has increased with growing volumes. In the RCT performed by Pokushalov and colleagues (44), thoracoscopic AF ablation using bipolar RF in mostly pAF patients resulted in more freedom from ATA recurrences than CA (81% vs. 47%, P=0.004). Comparable to safety outcomes in the FAST trial, the occurrence of severe adverse events was significantly higher in the surgical compared to the catheter arm (22% vs. 3%, P=0.02). Another RCT performed by Wang et al. (45) compared efficacy and safety results following thoracoscopic AF ablation using bipolar RF and CA, and included only patients with pAF. After twelve months, 89% of patients undergoing surgical ablation were free from ATA recurrences off AADs compared to 28% in the catheter arm (P=0.028). Finally, Haldar et al. (46) randomized persAF patients between thoracoscopic AF ablation using bipolar RF or CA. Remarkably, this RCT did not show superior rhythm outcomes for surgical ablation compared to CA (26% vs. 28%, P=0.83) and even results for CA were lower than reported in other studies (33,47). A potential explanation is that in the CASA-AF trial, linear lesions were performed with an outdated RF pen instead of the currently used linear RF device. Also, patients in the catheter arm underwent additional ablation of the mitral and cavo-tricuspid isthmus, whereas patients in the surgical arm did not. Moreover, limited experience was observed in the surgical arm.
Table 2
| Trial name | First author | Year | Lesions | Number of patients | pAF vs. persAF |
Efficacy allowing AAD 12 months | Efficacy off AAD 12 months |
Complications 12 months |
|---|---|---|---|---|---|---|---|---|
| Thoracoscopic versus catheter | ||||||||
| FAST | Boersma (19) | 2012 | SA: thoracoscopic RF PVI + GP + LAA + lines | SA n=61 | SA 74%/26% | 79% vs. 43%, P=0.001 | 66% vs. 37%, P=0.002 | 34% vs. 16%, P=0.027 |
| CA: PVI + lines | CA n=63 | CA 59%/41% | ||||||
| Pokushalov (44) | 2013 | SA: thoracoscopic RF PVI + box + GP + LAA | SA n=32 | SA 63%/37% | – | 81% vs. 47%, P=0.004 | 22% vs. 3%, P=0.02 | |
| CA: PVI + lines | CA n=32 | CA 56%/44% | ||||||
| Wang (45) | 2014 | SA: thoracoscopic RF PVI + GP + LAA + LOM | SA n=66 | SA 100%/0% | – | 89% vs. 75%, P=0.028 | – | |
| CA: PVI | CA n=72 | CA 100%/0% | ||||||
| CASA-AF | Haldar (46) | 2020 | SA: thoracoscopic RF PVI + GP + box + LAA | SA n=60 | SA 0/100% | – | 26% vs. 28%, P=0.83 | 18% vs. 15%, P=0.650 |
| CA: PVI + box + lines | CA n=60 | CA 0/100% | ||||||
| Hybrid versus catheter | ||||||||
| HARTCAP-AF | van der Heijden (33) | 2023 | HA: thoracoscopic RF PVI + box + LAA + lines | HA n=19 | HA 0/100% | 95% vs. 41%, P=0.001 | 89% vs. 36%, P=0.001 | 1% vs. 1%, P>0.99 |
| CA: PVI + box + lines | CA n=21 | CA 0/100% | ||||||
| CEASE-AF | Doll (47) | 2023 | HA: thoracoscopic RF PVI + box + LAA + lines | HA n=102 | HA 0/100% | 75% vs. 41%, P<0.001 | 63% vs. 35%, P=0.002 | 7.8% vs. 5.8%, P=0.751 |
| CA: PVI +option additional lines | CA n=52 | CA 0/100% | ||||||
| HALT-AF NCT05411614 | Ongoing | – | HA: subxiphoidal convergent + LAA + endocardial completion | HA n=50 | HA 0/100% | – | – | – |
| CA: standard CA | CA n=50 | CA 0/100% | ||||||
AF, atrial fibrillation; pAF, paroxysmal AF; persAF, persistent AF; AAD, antiarrhythmic drugs; SA, surgical ablation; RF, radiofrequency; PVI, pulmonary vein isolation; GP, ganglionated plexi; LAA, left atrial appendage; CA, catheter ablation; LOM, ligament of Marshall; HA, hybrid ablation.
All in all, these trials show us that in standalone thoracoscopic AF ablation, apart from PVI, it remains difficult to acquire complete linear lesions in all patients without endocardial validation and touch-up. Also, the plethora of different additional ablation strategies (Table 2) in both groups makes comparisons between standalone thoracoscopic AF ablation and CA, or even outcomes of standalone thoracoscopic AF ablation between trials challenging.
Hybrid vs. catheter ablation
The first RCT that evaluated the therapeutic efficacy and safety outcomes between thoracoscopic hybrid AF ablation and percutaneous CA alone is the HARTCAP-AF trial (33). In this trial, 41 ablation naïve patients with longstanding persAF were randomized between single-staged thoracoscopic hybrid AF ablation (n=19) or percutaneous CA (n=22). At twelve months after the procedure, freedom from ATA off AADs was significantly higher in the hybrid arm compared to the catheter arm (89% vs. 41%, P=0.002), without an increase in the number of serious adverse events (P=0.685). The strengths of this trial are that only ablation-naïve patients were included, it was a superiority design, the basic lesion set was equal in both groups and there was no difference in minor and major complications between the hybrid and the catheter arm. Interestingly, 8 (42%) patients in the hybrid arm required endocardial touch-up after surgical epicardial ablation, stressing the complementary importance of the endocardial EP validation and subsequent touch-up of acute conduction gaps. More recently, another RCT comparing thoracoscopic hybrid AF ablation with CA (CEASE-AF) in patients with longstanding persAF was published (47). This multi-center trial randomized 154 patients to hybrid or catheter ablation in a 2:1 fashion. At twelve months, freedom of ATA recurrences or increased doses of previously failed AADs was 71.6% in the hybrid arm versus 39.2% in the catheter arm (P<0.001). Also in this trial, there were no difference in safety outcomes between hybrid ablation and CA (7.8% vs. 5.8% respectively, P=0.751). Both trials strongly support the efficacy and safety of thoracoscopic hybrid AF ablation in patients with longstanding persAF (33,47) and should trigger treating physicians to at least discuss this option with patients when rhythm control is pursued.
The RCT that compared subxiphoid hybrid AF ablation with CA (CONVERGE trial), freedom of ATA recurrence off AAD was significantly better in the hybrid convergent arm than in the catheter arm (53.5% vs. 32.0% respectively, P=0.013), but the arrhythmia-free survival at one year was low compared to the HARTCAP-AF and CEASE-AF trial (30,33,47). An interesting ongoing RCT is the HALT-AF trial (NCT05411614) as it randomizes between subxiphoid hybrid AF ablation combined with LAA exclusion versus CA.
Efficacy and safety of hybrid AF ablation in real-world data
To provide a provisional overview of real-world long-term hybrid AF ablation outcomes to accompany this narrative review, we performed a systematic search of three electronic databases (PubMed, Embase, Cochrane Library) containing the following criteria: ‘thoracoscopic ablation’, ‘hybrid ablation’, ‘atrial fibrillation’ and all other possible alternative spelling. Articles were included for the pooled analysis of freedom from ATA recurrence when reporting on (I) consecutive patients undergoing thoracoscopic hybrid ablation using (II) Kaplan-Meier (K-M) survival analysis (see online Appendix 1 for more details). By selecting only articles with consecutive patients, we avoid the potential bias of reporting on a ’selected’ patient population as might be the case in RCTs or in meta-analyses including RCTs.
A total of seven studies were included in this analysis (49-55), five studies reported freedom from ATA recurrence allowing AAD (49,51,53-55) and five studies reported freedom from ATA recurrence off AAD (50-53,55). The resulting K-M curve representing the real-world outcome after hybrid AF ablation on and off AAD is presented in Figure 6. In this analysis, freedom from ATA allowing AAD was 83.7%±2.2%, 72.9%±2.9% and 71.7%±3.1% at 12, 24 and 36 months respectively, and 67.5%±2.0%, 61.2%±2.2% and 59.0%±2.5% off AAD at 12, 24 and 36 months respectively. Major complications were analyzed as well and were low: conversion to sternotomy 0.7%, re-thoracotomy 1.1%, stroke 0.5%, bleeding 1.6% and pacemaker implantation 1.0% (Table 3). It is encouraging to see that the good efficacy and safety outcomes of the hybrid AF ablation reported in meta-analysis and RCTs is confirmed in a real-world population.
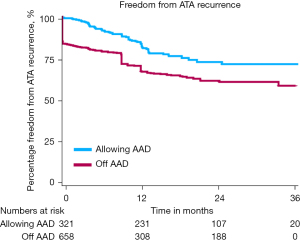
Table 3
| Study | Number of patients | Conversion | Rethoracotomy | Stroke | Bleeding | PM implant |
|---|---|---|---|---|---|---|
| de Asmundis et al. (49) | 51 | 0 | NR | 0 | 0 | 0 |
| Dunnington et al. (50) | 455 | 2 | 4 | 2 | NR | 7 |
| Lapenna et al. (51) | 50 | 0 | 2 | 1 | NR | 0 |
| Maesen et al. (52) | 64 | 0 | NR | 0 | 0 | 0 |
| Mahapatra et al. (53) | 15 | NR | NR | 0 | 0 | NR |
| Pong et al. (54) | 84 | 4 | NR | 1 | NR | 0 |
| van der Heijden et al. (55) | 119 | 0 | 1 | 0 | 4 | 1 |
| Pooled data (%) | 838 | 0.7 | 1.1 | 0.5 | 1.6 | 1.0 |
| 95% CI (%) | – | 0.3–1.6 | 0.5–2.3 | 0.2–1.2 | 0.6–4.1 | 0.5–1.9 |
AF, atrial fibrillation; PM, pacemaker implantation; NR, not reported; CI, confidence interval.
The “Maastricht approach”
In Maastricht, the close collaboration between a dedicated heart and research team has led to the development of several surgical ablation pathways to further individualize treatment strategies (Figure 7). First, all patients with AF are discussed in the heart team consisting of an experienced EP and a cardiac surgeon. Patients that are eligible for minimally invasive surgical AF ablation can then undergo a variety of treatment options, depending on pre-operative AF type, comorbidities, patient characteristics, logistical and/or safety reasons and the preference of the patient and physician. Importantly, treatment pathways for patients that undergo AF ablation via sternotomy or CA as first line therapy are out of scope for the current review.
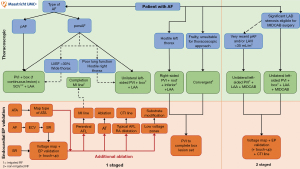
Depending on pre-operative AF type (pAF or persAF), either a unilateral left-sided thoracoscopic or a bilateral thoracoscopic approach can be performed. For patients with pAF, the preferred strategy is a bilateral approach consisting of PVI and box isolation in one continuous lesion using irrigated RF, followed by LAA management and SCV isolation. We believe SCV isolation is important in this patient category, as patients with pAF often present with trigger-initiated AF rather than substrate perpetuated AF. For patients presenting with persAF, depending on the preference of the surgeon, logistical and/or safety reasons, either a unilateral left-sided thoracoscopic or bilateral thoracoscopic approach is performed. Specific reasons to opt for a bilateral approach over a unilateral approach are a poor left ventricular ejection fraction (<30%) or a wide thorax, as handling the instruments below the heart during a unilateral left-sided approach might trigger ventricular arrhythmias. In the case of a wide thorax, complete encircling of the right PVs (especially the RSPV) can be challenging when going unilaterally left-sided. On the other hand, a poor lung function or a hostile right thorax are reasons to prefer a unilateral left-sided thoracoscopic over a bilateral approach.
Immediately after the surgical procedure, the endocardial procedure is performed. Although both single-staged and two-staged hybrid procedures have their own advantages, we prefer a single-staged hybrid procedure, mainly due to close cooperation with the EP (Figure 4). If the patient is in AF at the end of the surgical procedure, an electrical cardioversion is usually performed to restore SR. Extensive voltage mapping and testing of entrance and exit block (EP validation) is then performed and targeted endocardial touch-up ablation can be performed, if necessary. For patients with a history of an ATA on ECG, an AT induction protocol is performed. If inducible, the type of ATA is mapped and treated accordingly. In the case of a peri-mitral flutter, a mitral isthmus line is created from the endocardium and, if needed, completed by the cardiac surgeon from the epicardium during a single staged hybrid procedure. Patients presenting with a typical right atrial flutter or dilatation undergo additional ablation of the cavo-tricuspid isthmus. Finally, if low voltage zones are mapped, additional endocardial substrate modification is performed.
Moreover, patients with AF that are also known to have significant disease of the left anterior descending (LAD) artery suitable for MIDCAB surgery (and optional staged revascularization in the case of multivessel disease), can undergo a unilateral left-sided thoracoscopic AF ablation, consisting of PVI, box isolation and LAA exclusion, concomitant with MIDCAB surgery. Only in the case of very recently diagnosed pAF and/or a small LAVI (<35 mL/m2), only bilateral PVI will be performed. Not only does PVI alone result in satisfactory outcomes for patients with pAF and small atria, but this way the healthy atrial contractile tissue can partially be preserved. In a recent cohort study including all consecutive patients undergoing this all-in-one minimally invasive approach for patients with AF and LAD disease suitable for MIDCAB surgery in the MUMC+, the procedure was both efficacious, safe and thus represents a valid alternative to sternotomy (56). After six months, a staged endocardial EP validation and if necessary, touch-up ablation and additional ablation of lines is performed. Of course, future studies evaluating the efficacy and safety outcomes of a larger cohort will be needed in order to compare these outcomes with patients that underwent bypass grafting and AF ablation via sternotomy.
Additionally, a unilateral right-sided thoracoscopic approach may be suitable for patients with a hostile left thorax, although endocardial ablation of the left PVs and potential touch-up of the roof and inferior lines is necessary to complete isolation of the box. Finally, the convergent technique is considered a useful hybrid option for frail patients that are unsuitable for a thoracoscopic approach.
Future perspectives
Although a thoracoscopic hybrid AF ablation approach seems promising when performed by a dedicated team, certain procedural aspects of the hybrid AF procedure remain to be further investigated. For example, it is still unknown how transmurality based on technical and/or patient specific aspects can be predicted, or how aggressive the RF ablation should be to obtain transmurality of the lesions without fully compromising normal LA function. Other elements for optimization include further patient selection, the selection of anatomical or structural ablation targets and the prediction of which strategy to use at what time for which patient. In our opinion, in order to gain further insights in these gaps in knowledge and to further improve outcomes, the hybrid AF ablation procedure should preferably only be performed in centers with substantial exposure to AF patients that are referred for minimally invasive surgical treatment. To reduce the influence of the learning curve in the acquisition of advanced skills in thoracoscopy, centralization of such a specialized treatment option to hospitals that have a dedicated team involved in standardized and integrated pre- and post-clinical care and research pathways could play an important role in the further optimization of outcomes following hybrid ablation. A good example of combining clinical work with dedicated research is the ISOLATION study; a prospective, multicenter cohort study in the MUMC+ and the Radboud University Medical Centre that aims to identify predictors of successful AF ablation from clinical factors, AF patterns, anatomical and electrophysiological characteristics, circulating biomarkers and genetic background (57). Additionally, lifestyle modification and risk management is being implemented as well in order to further optimize outcomes following hybrid ablation.
Finally, future studies that report on other important endpoints besides rhythm outcome, such as cost-effectiveness, short- and long-term quality of life (overall well-being but also disease specific) and the social and economic impact of a hybrid procedure due to hospitalizations, stroke and mortality, are needed as well in order to obtain a more holistic view of success following hybrid AF ablation.
Conclusions and take-home messages
Given the underlying complex pathophysiology of AF, especially in more persistent forms, it remains challenging to tailor rhythm control therapy in an individualized fashion. The hybrid AF ablation was designed to address this goal by combining the strengths of an epicardial surgical approach with that of a percutaneous endocardial approach. This review summarizes the rationale, the technical aspects, the possibilities to tailor the ablation and the efficacy and safety outcomes of this approach. Given the consistently good efficacy outcomes and the reliable safety profile of this procedure in experienced centers, we believe that in ablation naïve patients with persistent forms of AF, hybrid AF ablation should be considered and discussed with our patients as an alternative to percutaneous procedures. After all, it is the patient that undergoes the therapy, not the physician.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: B.M. is a consultant for AtriCure and Medtronic. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265-325. [Crossref] [PubMed]
- Maesen B, Verheule S, Zeemering S, et al. Endomysial fibrosis, rather than overall connective tissue content, is the main determinant of conduction disturbances in human atrial fibrillation. Europace 2022;24:1015-24. [Crossref] [PubMed]
- Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J 2017;38:1294-302. [Crossref] [PubMed]
- Verheule S, Eckstein J, Linz D, et al. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog Biophys Mol Biol 2014;115:173-85. [Crossref] [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122:109-18. [Crossref] [PubMed]
- de Groot NM, Houben RP, Smeets JL, et al. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation 2010;122:1674-82. [Crossref] [PubMed]
- Maesen B, Zeemering S, Afonso C, et al. Rearrangement of atrial bundle architecture and consequent changes in anisotropy of conduction constitute the 3-dimensional substrate for atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6:967-75. [Crossref] [PubMed]
- Ho SY, Sánchez-Quintana D. The importance of atrial structure and fibers. Clin Anat 2009;22:52-63. [Crossref] [PubMed]
- Nault I, Miyazaki S, Forclaz A, et al. Drugs vs. ablation for the treatment of atrial fibrillation: the evidence supporting catheter ablation. Eur Heart J 2010;31:1046-54. [Crossref] [PubMed]
- Maesen B, Van-Loo I, Pison L, et al. Surgical Ablation of Atrial Fibrillation: is Electrical Isolation of the Pulmonary Veins a Must? J Atr Fibrillation 2016;9:1426. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. Correction appears in Eur Heart J 2021;42:507; correction appears in Eur Heart J 2021;42:546-7; correction appears in Eur Heart J 2021;42:4194.
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812-22. [Crossref] [PubMed]
- Kistler PM, Chieng D, Sugumar H, et al. Effect of Catheter Ablation Using Pulmonary Vein Isolation With vs Without Posterior Left Atrial Wall Isolation on Atrial Arrhythmia Recurrence in Patients With Persistent Atrial Fibrillation: The CAPLA Randomized Clinical Trial. JAMA 2023;329:127-35. [Crossref] [PubMed]
- Valderrábano M, Peterson LE, Swarup V, et al. Effect of Catheter Ablation With Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation: The VENUS Randomized Clinical Trial. JAMA 2020;324:1620-8. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J Am Coll Cardiol 2016;68:1929-40. [Crossref] [PubMed]
- De Potter T, Reddy V, Neuzil P, et al. Acute safety and performance outcomes from the inspIRE trial using a novel pulsed field ablation system for the treatment of paroxysmal atrial fibrillation. Eur Heart J 2021;42:ehab724.0380.
- Cox JL, Schuessler RB, D'Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83.
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [Crossref] [PubMed]
- Pison L, La Meir M, van Opstal J, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol 2012;60:54-61. [Crossref] [PubMed]
- Vroomen M, Pison L. Hybrid ablation for atrial fibrillation: a systematic review. J Interv Card Electrophysiol 2016;47:265-74. [Crossref] [PubMed]
- Maesen B, Luermans JGLM, Bidar E, et al. A hybrid approach to complex arrhythmias. Europace 2021;23:ii28-33. [Crossref] [PubMed]
- Bugge E, Nicholson IA, Thomas SP. Comparison of bipolar and unipolar radiofrequency ablation in an in vivo experimental model. Eur J Cardiothorac Surg 2005;28:76-80; discussion 80-2. [Crossref] [PubMed]
- Matteucci F, Maesen B, De Asmundis C, et al. Comparison between biparietal bipolar and uniparietal bipolar radio frequency ablation techniques in a simultaneous procedural setting. J Interv Card Electrophysiol 2021;61:567-75. [Crossref] [PubMed]
- Nakagawa H, Yamanashi WS, Pitha JV, et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation 1995;91:2264-73. [Crossref] [PubMed]
- Bijvoet GP, Chaldoupi SM, Bidar E, et al. Epicardial box lesion using bipolar biparietal radiofrequency and multimodality scar evaluation-a case series. Eur Heart J Case Rep 2022;6:ytab530. [Crossref] [PubMed]
- Maesen B, La Meir M. Unilateral Left-sided Thoracoscopic Ablation of Atrial Fibrillation. Ann Thorac Surg 2020;110:e63-6. [Crossref] [PubMed]
- Fleerakkers J, Hofman FN, van Putte BP. Totally thoracoscopic ablation: a unilateral right-sided approach. Eur J Cardiothorac Surg 2020;58:1088-90. [Crossref] [PubMed]
- Vos LM, Fleerakkers J, Hofman FN, et al. Closed-chest unilateral thoracoscopic ablation: box lesion with radiofrequency clamps only. Eur J Cardiothorac Surg 2022;62:ezac316. [Crossref] [PubMed]
- DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ Arrhythm Electrophysiol 2020;13:e009288. [Crossref] [PubMed]
- Toale C, Fitzmaurice GJ, Eaton D, et al. Outcomes of left atrial appendage occlusion using the AtriClip device: a systematic review. Interact Cardiovasc Thorac Surg 2019;29:655-62. [Crossref] [PubMed]
- Vroomen M, Maesen B, Luermans JL, et al. Epicardial and Endocardial Validation of Conduction Block After Thoracoscopic Epicardial Ablation of Atrial Fibrillation. Innovations (Phila) 2020;15:525-31. [Crossref] [PubMed]
- van der Heijden CAJ, Weberndörfer V, Vroomen M, et al. Hybrid Ablation Versus Repeated Catheter Ablation in Persistent Atrial Fibrillation: A Randomized Controlled Trial. JACC Clin Electrophysiol 2023;9:1013-23. [Crossref] [PubMed]
- Baudo M, Petruccelli RD, D'Alonzo M, et al. Rhythm outcomes of minimally-invasive off-pump surgical versus catheter ablation in atrial fibrillation: A meta-analysis of reconstructed time-to-event data. Int J Cardiol 2023;376:62-75. [Crossref] [PubMed]
- Bisleri G, Pandey AK, Verma S, et al. Combined Minimally Invasive Surgical and Percutaneous Catheter Ablation of Atrial Fibrillation: JACC Review Topic of the Week. J Am Coll Cardiol 2023;81:606-19. [Crossref] [PubMed]
- Jiang YQ, Tian Y, Zeng LJ, et al. The safety and efficacy of hybrid ablation for the treatment of atrial fibrillation: A meta-analysis. PLoS One 2018;13:e0190170. [Crossref] [PubMed]
- Pearman CM, Poon SS, Bonnett LJ, et al. Minimally Invasive Epicardial Surgical Ablation Alone Versus Hybrid Ablation for Atrial Fibrillation: A Systematic Review and Meta-Analysis. Arrhythm Electrophysiol Rev 2017;6:202-9. [Crossref] [PubMed]
- Syed FF, Oral H. Electrophysiological Perspectives on Hybrid Ablation of Atrial Fibrillation. J Atr Fibrillation 2015;8:1290. [Crossref] [PubMed]
- Je HG, Shuman DJ, Ad N. A systematic review of minimally invasive surgical treatment for atrial fibrillation: a comparison of the Cox-Maze procedure, beating-heart epicardial ablation, and the hybrid procedure on safety and efficacy. Eur J Cardiothorac Surg 2015;48:531-40; discussion 540-1. [Crossref] [PubMed]
- van der Heijden CAJ, Vroomen M, Luermans JG, et al. Hybrid versus catheter ablation in patients with persistent and longstanding persistent atrial fibrillation: a systematic review and meta-analysis†. Eur J Cardiothorac Surg 2019;56:433-43. [Crossref] [PubMed]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;384:2081-91. [Crossref] [PubMed]
- Mhanna M, Beran A, Al-Abdouh A, et al. Hybrid convergent ablation versus endocardial catheter ablation for atrial fibrillation: A systematic review and meta-analysis. J Arrhythm 2021;37:1459-67. [Crossref] [PubMed]
- Edgerton Z, Perini AP, Horton R, et al. Hybrid Procedure (Endo/Epicardial) versus Standard Manual Ablation in Patients Undergoing Ablation of Longstanding Persistent Atrial Fibrillation: Results from a Single Center. J Cardiovasc Electrophysiol 2016;27:524-30. [Crossref] [PubMed]
- Pokushalov E, Romanov A, Elesin D, et al. Catheter versus surgical ablation of atrial fibrillation after a failed initial pulmonary vein isolation procedure: a randomized controlled trial. J Cardiovasc Electrophysiol 2013;24:1338-43. [Crossref] [PubMed]
- Wang S, Liu L, Zou C. Comparative study of video-assisted thoracoscopic surgery ablation and radiofrequency catheter ablation on treating paroxysmal atrial fibrillation: a randomized, controlled short-term trial. Chin Med J (Engl) 2014;127:2567-70.
- Haldar S, Khan HR, Boyalla V, et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: CASA-AF randomized controlled trial. Eur Heart J 2020;41:4471-80. [Crossref] [PubMed]
- Doll N, Weimar T, Kosior DA, et al. Efficacy and safety of hybrid epicardial and endocardial ablation versus endocardial ablation in patients with persistent and longstanding persistent atrial fibrillation: a randomised, controlled trial. EClinicalMedicine 2023;61:102052. [Crossref] [PubMed]
- Castellá M, Kotecha D, van Laar C, et al. Thoracoscopic vs. catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. Europace 2019;21:746-53. [Crossref] [PubMed]
- de Asmundis C, Varnavas V, Sieira J, et al. Two-year follow-up of one-stage left unilateral thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation. J Interv Card Electrophysiol 2020;58:333-43. [Crossref] [PubMed]
- Dunnington GH, Pierce CL, Eisenberg S, et al. A heart-team hybrid approach for atrial fibrillation: a single-centre long-term clinical outcome cohort study. Eur J Cardiothorac Surg 2021;60:1343-50. [Crossref] [PubMed]
- Lapenna E, Cireddu M, Nisi T, et al. Heart-team hybrid approach to persistent atrial fibrillation with dilated atria: the added value of continuous rhythm monitoring. Eur J Cardiothorac Surg 2021;60:222-30. [Crossref] [PubMed]
- Maesen B, Pison L, Vroomen M, et al. Three-year follow-up of hybrid ablation for atrial fibrillation. Eur J Cardiothorac Surg 2018;53:i26-32. [Crossref] [PubMed]
- Mahapatra S, LaPar DJ, Kamath S, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91:1890-8. [Crossref] [PubMed]
- Pong T, Shah RL, Carlton C, et al. Hybrid Ablation for Atrial Fibrillation: Safety & Efficacy of Unilateral Epicardial Access. Semin Thorac Cardiovasc Surg 2023;35:277-86. [Crossref] [PubMed]
- van der Heijden CAJ, Weberndörfer V, Luermans JGLM, et al. Hybrid ablation of atrial fibrillation: A unilateral left-sided thoracoscopic approach. J Card Surg 2022;37:4630-8. [Crossref] [PubMed]
- van der Heijden CAJ, Segers P, Masud A, et al. Unilateral left-sided thoracoscopic ablation of atrial fibrillation concomitant to minimally invasive bypass grafting of the left anterior descending artery. Eur J Cardiothorac Surg 2022;62:ezac409. [Crossref] [PubMed]
- Verhaert DVM, Linz D, Chaldoupi SM, et al. Rationale and Design of the ISOLATION Study: A Multicenter Prospective Cohort Study Identifying Predictors for Successful Atrial Fibrillation Ablation in an Integrated Clinical Care and Research Pathway. Front Cardiovasc Med 2022;9:879139. [Crossref] [PubMed]






