Hybrid strategies for stand-alone surgical ablation of atrial fibrillation
Introduction
Atrial fibrillation (AF) represents a growing clinical challenge for the healthcare system affecting more than 30 million patients worldwide with an increasing prevalence estimated to reach up to 16 million people in the U.S. alone by 2050 (1).
Furthermore, AF is reported to be a major cause of cardiac morbidity and mortality and significantly reduces the quality of life in symptomatic patients (2).
The most recently updated guidelines recommend antiarrhythmic drugs and catheter ablation (CA) as first line therapy (Class I) in AF patients, while surgical ablation, either stand-alone or hybrid, is recommended (Class IIa) for AF patients refractory to medical therapy or who failed CAs (3).
Despite CA showing satisfactory results in patients with paroxysmal AF, limited efficacy has been reported for patients with non-paroxysmal AF (either persistent or long-standing persistent AF) where a more complex electro-anatomical substrate sustains recurrences. Nevertheless, the most remarkable advantage of percutaneous ablations relies on the capability to endocardially check transmurality and complete ablation gaps by mapping the activation and propagation waves and performing lesions otherwise not accessible epicardially during off-pump minimally invasive techniques (4).
To date, the Cox-Maze procedure is still considered the most effective treatment for AF providing excellent results in terms of stable sinus rhythm restoration at long-term follow-up. Core principle of this technique relies on both the necessity to confine the triggering activities originating from the four pulmonary veins (PVs) and to interrupt micro/macro re-entrant circuits at the level of the right and left atrium sustaining and perpetuating AF. This technique allows a direct visualization of anatomical structures and the ability to perform stable continuous transmural lesions (5).
In its latest iteration, the Cox-Maze IV, cut-and-sew lines have been replaced by linear radiofrequency or cryothermy ablations. However, this technique remained under-performed because of its invasiveness and higher risk of perioperative complications (6,7). Over the years, the suboptimal results of CA in non-paroxysmal AF, the advancement in surgical technology and the growing experience in minimally invasive thoracoscopic off-pump techniques in arrhythmia surgery have led to a quest for new approaches for the treatment of AF. In this context, the concept of a “hybrid” procedure for the treatment of AF was developed, combining the advantages of both thoracoscopic and CA procedures and potentially limiting disadvantages and complications.
We aim to provide an updated overview on the rationale behind the concept of hybrid ablation for the treatment of AF with regard to different available strategies, results and expert opinions.
Hybrid treatment of AF
Transcatheter endocardial AF ablation
Endocardial CA for the treatment of stand-alone AF is the first-line strategy in symptomatic patients according to the most recent guidelines (3).
PV ectopy is the main trigger in 90% of patients with paroxysmal AF while the remaining 10% have been recognized at the level of the coronary sinus, crista terminalis and superior vena cava (8). Thus, CA by means of PV isolation showed good results in terms of stable sinus rhythm restoration in patients with paroxysmal AF with a success rate around 70% at 1 year. However, the efficacy of endocardial ablation in persistent and long-standing persistent AF is still far from being satisfactory (9-12).
Besides, electroanatomical mapping data in patients with non-paroxysmal AF showed that macro and micro re-entrant circuits are sustained by a more complex anatomical substrate, including electrical endo/epicardial inhomogeneity and fibrosis, thus partially explaining inconsistent results of endocardial CAs in this specific subset of patients (13,14).
However, during CA, electrophysiologists (EPs) can easily and effectively address triggers originating from the four PVs and eventually add “substrate specific ablations” with additional lesions at the level of the cavo-tricuspid isthmus, the coronary sinus and the mitral isthmus, which are otherwise not feasible during epicardial thoracoscopic ablation. Moreover, the opportunity of performing electroanatomical mapping during CA procedures has two main advantages: (I) to identify and perform ablations tackling non-PV-triggers; (II) to verify completeness in terms of transmurality of any ablation lines previously performed (either previous endocardial or epicardial ablation).
On the other hand, endocardial CA showed some limitations. First of all, the isolation of the LA posterior wall can be challenging: available catheters lack the possibility to perform continuous transmural linear lesions, thus hampering the interruption of re-entrant circuits at this level. Secondarily, the thermal spread caused by an extensive use of cryothermy and/or radiofrequency (RF) delivered endocardially, may induce thermogenic injury of the surrounding structures such as phrenic nerve and esophagus (15). Lastly, when LAA closure is performed endocardially, it only addresses stroke prevention without modifying the electrical triggering activity that might be useful in non-paroxysmal AF types (16).
New devices have been developed in order to overcome these limitations with promising results. Contact force sensing RF-guided ablation showed a lower rate of PV reconnection at the time of repeated procedures (17). In adjunct, more complex indexes, such as ablation index (AI) or lesion index (LI), have showed promising results with more than 90% of freedom from AF in paroxysmal AF patients after AI-guided ablation (18-20).
Moreover, other technologies, such as electroporation, showed a lower impact in damaging surrounding tissues through a tissue-selective action (21).
Rationale of surgical ablation
To date, the most reliable and effective long-term results for the treatment of AF have been achieved with the surgical Cox-Maze ablation set and its latest evolution, the so-called Cox-Maze IV. With this approach, 91% and 78% of patients showed stable sinus rhythm restoration at 5.4 years of follow-up with and without antiarrhythmic drugs (6,22).
The key aspect of this technique lies in the capability of performing effective transmural lesions encircling the four PVs thus limiting AF triggering activity, and then, interrupting re-entrant circuits, which are usually located around circular structures [left atrial appendage (LAA)—mitral annulus—superior and inferior vena cava—tricuspid annulus—coronary sinus—right atrial appendage; Figure 1] (23). However, the need of the cardiopulmonary bypass and the invasiveness of this technique, strongly limited a wide adoption of this procedure being nowadays performed concomitantly with other cardiac procedures. On the other hand, the consistency of its results in terms of stable sinus rhythm restoration at long-term follow-up have never been replicated by any other technique so, globally, the Cox-Maze lesions set are widely considered and remain the gold standard for AF treatment (24-28).
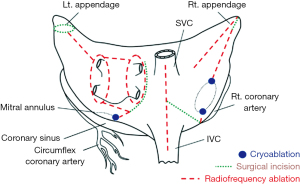
In the last decade, the introduction of minimally invasive thoracoscopic techniques re-launched interest towards arrhythmia surgery and in particular towards AF treatment by means of off-pump closed-chest procedures with the aim of limiting the surgical invasiveness and complications without hampering results in terms of stable conversion to sinus rhythm.
Thus, off-pump thoracoscopic procedures have been performed with the aim of replicating the more reliable Cox-Maze ablation lines by means of epicardial lesions alone (29,30).
Studies on surgical minimally invasive off-pump epicardial ablation for the treatment of stand-alone AF reported excellent results with an incidence of stable sinus rhythm restoration at long-term follow-up of 86.2% in the overall population and ranging from 79% to 52% off antiarrhythmic drugs (AADs) in paroxysmal and non-paroxysmal AF (31,32).
Although different techniques have been introduced in order to attain similar success rates that could be equivalent to those of the surgical Cox-Maze, these results are far from being considered comparable to “the gold-standard” technique. A major flaw is considered the impossibility of any minimally invasive thoracoscopic epicardial ablation procedure alone to exactly replicate the whole Cox-Maze lesions set, in particular at the level of the coronary sinus, the mitral and tricuspid isthmus, thus making these novel techniques incomplete.
Nevertheless, strengths include the possibility to perform a durable transmural box lesion encircling, in a continuous linear fashion, the four PVs and the posterior aspect of the LA (33). This lesion is considered the cornerstone of any ablation procedure and the success rate in terms of sinus rhythm restoration strictly relies on its complete transmurality (34). Moreover, during surgical ablation, surgeons have the unique opportunity of a direct visualization of cardiac structures, including the possibility to manage the LAA epicardially. This is of paramount importance since this structure should be managed for stroke prevention but also for electrical isolation, thus improving results of AF interventions (35). These aspects explain the higher success rates in terms of stable sinus rhythm restoration reported after surgical AF ablation when compared to CA (36).
An additional value of surgical ablation is the possibility to eventually divide the ligament of Marshall as well as to perform a connecting lesion between LAA and the box-lesion, thus interrupting macro re-entrant circuits that may develop around the ostium of the LAA (37).
Important, but not mandatory, is the possibility to directly complete the surgical ablation with epicardial lesions at the level of the right atrium (RA), in particular the intercaval line and the right atrial appendage (RAA) line involved in micro and macro re-entrant circuits sustaining AF while, as mentioned above, cavotricuspid isthmus ablation is not achievable epicardially during beating-heart procedures and it should be left to EPs (29).
Rationale of hybrid procedure
Considering strengths and flaws of both options, the “hybrid concept” aims to highlight and leverage advantages of both techniques while seeking to reduce perioperative complications without hampering long-term results in terms of stable sinus rhythm restoration. Although, the “ideal” hybrid procedure must adhere as much as possible to the Maze concept in order to attain the best possible rhythm outcomes (38).
Currently, hybrid strategies can be performed simultaneously in a joint setting of surgical and CA or staged, with minimally invasive thoracoscopic ablation performed first, then followed by a mandatory CA at a later stage.
Similar outcomes in terms of sinus rhythm restoration have been reported when endocardial CA has been performed simultaneously compared to a later staged approach (39,40).
The main advantage for patients who received surgical and CA sequentially is the possibility to quickly identify and treat immediately any lesion gaps, thus improving the chance to stably restore sinus rhythm and induce reverse remodeling. Moreover, both procedures are performed during the same hospitalization, thus potentially optimizing costs. However, tissue edema induced at the time of surgical ablation may result in a false transmural block that reverses once inflammation fades. Conversely, staged hybrid approaches performed after a variable timeframe of at least 45 days lead to lesion stabilization and scar formation, thus allowing for a more precise endocardial mapping and gaps investigation at the time of CA step. This aspect is of paramount importance and explains the current tendency to prefer a staged approach with thoracoscopic ablation carried out for first and then, trans-catheter approach performed following a blanking period.
In summary, when a hybrid strategy is performed, the objective of the surgical step is to create all the lines of the Maze procedure feasible with an epicardial approach. Thus, the box-lesion set addressing the four PVs and the LA posterior wall should be performed together with LAA epicardial exclusion and the treatment of the ligament of Marshall. Additionally, RA lines could be added. Furthermore, the subsequent CA, either during sequential or staged setting, should be performed with the aim to perform mapping, touch-up ablations, gaps closure and eventually treat cavo-tricuspid and mitral isthmus in case of induction of atrial flutter (41).
Surgical technique
With the most recent minimally invasive off-pump techniques, a successful epicardial ablation can be safely performed by means of: (I) the Fusion technique; (II) the bipolar clamp technique; and (III) with the most recent “convergent technique”.
The “Fusion” technique
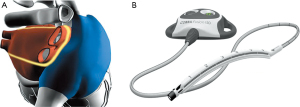
This approach foresees the use of a versapolar (mono and bipolar RF energy) vacuum-assisted suction device (Cobra Fusion, Atricure, West Chester, OH, USA). Usually through a unilateral right-sided thoracoscopic approach, this linear device is gently slid into the transverse and oblique sinuses after blunt dissection of the pericardial reflections at the level of the superior and inferior vena cava and after fat-pad removal at the level of the Waterstone’s groove and the roof of the left atrial (LA). Aim of this procedure is to perform a continuous linear ablation line encircling the four PVs “en-bloc” and the posterior aspect of the LA. Additionally, intercaval and right atrial appendage lines could be added at the end of the procedure. Due to the right thoracoscopic access, LAA and the ligament of Marshall management could be extremely challenging (Figure 2) (42).
The bipolar clamp technique
A specifically designed bipolar RF clamp is used, either during unilateral left-sided or bilateral thoracoscopic approach.
Briefly, during unilateral left-sided thoracoscopic ablation, left PVs first, then right PVs, are encircled by means of a dissection tool (Lumitip; Atricure, West Chester, OH, USA) followed by a bipolar RF clamp (Synergy System; Atricure). The aim of this technique is to provide antral isolation of the two couples of PVs (right and left), then connected by means of connecting lesions at the level of the roof and the floor of the LA in order to isolate the posterior aspect of the LA and create a box-lesion. Linear connections are performed by means of the Coolrail RF device (Atricure) (43).
Similarly, during the bilateral thoracoscopic approach, dedicated right and left shaped clamps are available in order to achieve PVs isolation of the right and left PVs while connecting lesions (Coolrail; Atricure) at the level of the roof and the floor of the LA are performed with a linear bipolar device in order to exclude the posterior aspect of the LA. Conversely, a different specifically designed irrigated bipolar RF device allows the surgeon to perform overlapping lesions sequentially encircling en-block PVs and the LA posterior wall through a bilateral thoracoscopic approach either (Medtronic Cardioblate Gemini, Medtronic, Minneapolis, MN, USA).
In both instances, LAA can be easily managed and excluded epicardially and the ligament of Marshall divided. The connecting lesion between left superior PV and the LAA can be helpful in order to improve clinical results. Lastly, right-sided lesions at the level of the superior and inferior vena cava can be easily accessible, thus mimicking MAZE lesions (30).
The “hybrid convergent” technique
Through a subxiphoid access, a specifically designed vacuum assisted unipolar RF probe is inserted into the pericardium in order to perform multiple contiguous and parallel lesions across the LA posterior wall under pericardioscopic guidance. The aim of this technique is to fully isolate the LA posterior wall and the proximity of the antra of the four PVs while leaving to EPs to complete PVs ablation endocardially in a simultaneous single stage setting in a hybrid room or in a staged approach. The ablation catheter has undergone numerous modifications, from the Visitrax and Numeris guided coagulation devices used in earlier studies to the fourth-generation EPi-Sense (EPi-Sense Guided Coagulation System, AtriCure, Inc., Mason, OH, USA), featuring sensing function that can determine the type of tissue in contact. It is highly recommended to monitor the esophageal temperature and use saline irrigation to reduce adjacent tissue heating. The procedure evolved from an extracardiac maze approach with extensive epicardial ablation to the creation of linear parallel overlapping lesions on the posterior wall (Figure 3) (44).

LAA management
While LAA could be managed either by means of percutaneous endocardial approach or by means of epicardial surgical occlusion, only this latter approach is able to reach either effective LAA exclusion, thus reducing the risk of stroke and immediate electrical isolation, thus reducing AF recurrences (16,45,46).
Based on this evidence, LAA should always be managed during AF treatment regardless of the type of the hybrid procedure performed. Moreover, due to its capability to definitely interrupt LAA triggering activity, epicardial closure should be preferred over endocardial closure techniques.
To date, there is only one available device specifically designed for minimally invasive epicardial surgical closure (AtriClip PRO2; Atricure).
Catheter ablation
The access to the LA is achieved through a trans-septal puncture via transvenous femoral access. Under systemic heparinization, electroanatomical mapping is performed by means of multipolar mapping under guidance with a 3D mapping system. It is possible to map and identify any surgical ablation lines or pre-existing CA lines that can be checked for gaps. In addition, non-PVI triggers can be easily studied and detected. Ablation is then performed by means of different energies: radiofrequency catheters with a force-irradiated tip or with a cryoballoon probe that targets the ostium of the four PVs. Recently, new types of energy have become available, such as electroporation. Lastly, through RF, additional ablation lines can be performed endocardially in case of a documented atrial flutter or induced atrial flutter during the ablation (cavo-tricuspid or mitral isthmus line).
Discussion
While CA showed promising results for the treatment of paroxysmal AF with a success rate reaching 70% of stable sinus rhythm restoration at 1 year, the more complex electrophysiological substrate in persistent and long-standing persistent AF highlighted the inadequacy of this approach due to the high incidence of arrhythmia recurrences after single and multiple consecutive ablations (47,48). On the other hand, minimally invasive thoracoscopic ablation procedures significantly reduced invasiveness, showed a high safety and effectiveness profile and reported intriguing results in both paroxysmal and non-paroxysmal AF. Therefore, the “hybrid” ablation concept has been developed to take advantage of both thoracoscopic ablation procedures and CA to attain the best possible outcome in the treatment of AF while minimizing invasiveness and operative complications.
Overall, the experience from different groups performing hybrid ablation demonstrated that this strategy is safe and effective when compared to CA alone in patients with non-paroxysmal AF. In a recent meta-analysis of 22 studies, Varzaly et al., showed a global stability to sinus rhythm maintenance of 79% and 71% with or without AADs at a mean follow-up of 19 months. Authors included hybrid AF ablations regardless of the timing of the hybrid procedures (concomitant or staged). Of note, most of the patients (89%) had non-paroxysmal AF (39). This meta-analysis is consistent with findings reported by Richardson et al., where timing of the CA (staged or simultaneous approach) does not affect clinical outcomes in terms of sinus rhythm restoration while, conversely, LAA exclusion when performed, was associated with higher rates of sinus rhythm restoration off AAD (40).
It is well-known that the inclusion of unmatched retrospective data into meta-analyses may jeopardize the results. Therefore, a recent meta-analysis analyzed the rhythm outcomes of hybrid ablation vs. CA in only randomized-controlled and propensity-matched studies (49). Authors confirmed how the hybrid group had significantly higher rates of freedom from AF with [odds ratio (OR) =2.78; 95% confidence interval (CI): 1.82–4.24; P<0.001] or without AAD (OR =2.75; 95% CI: 1.63–4.65; P<0.001) than the CA group. Complication rates were more frequent in the hybrid group (9.4% vs. 1.6%; P<0.001), comparable with the meta-analysis by Varzaly et al. (39).
Recently, sinus rhythm restoration was investigated in a cohort of patients with non-paroxysmal AF without prior ablations randomized to single-stage hybrid ablation vs. CA alone. Authors reported a higher success rate in patients undergoing hybrid ablation (hybrid: 89% vs. CA: 41%; P=0.002) (50). Previously, single-stage hybrid ablation studies have reported a success rate of 90% and 82% of sinus rhythm maintenance in persistent AF (51,52).
Thus, by means of a simultaneous CA hybrid approach, ablation gaps can immediately be identified and treated. Mitral or cavo-tricuspid isthmuses, that are more easily accessible endocardially, can be ablated without increasing the risk of pacemaker implantation (53).
Others described the adoption of a staged hybrid AF ablation yielded to similar results. Bulava et al. reported a freedom from atrial arrhythmia off AADs of 94% and 84% on AADs at 1-year follow-up (54). Muneretto et al. (HISTORIC-AF trial) described the feasibility of a hybrid right unilateral thoracoscopic approach (Fusion technique) in persistent AF patients. At 12-month follow-up, sinus rhythm was achieved in 88% of patients (42).
Regarding the controversies about the timing of the hybrid procedures, Richardson et al. analyzed the effects of the hybrid staging strategy on AF recurrence (40). In their study, 52 patients underwent simultaneous ablation, while 31 received staged hybrid AF ablation. Although the staged strategy significantly increased the diagnosis of ablation gaps (OR =6; 95% CI: 2–17; P=0.001), it did not improve the time to first AF recurrence [hazard ratio (HR) =1.0; 95% CI: 0.4–2.4; P=0.9]. This is in line with a recent study by Nasso and colleagues suggesting similar outcomes between the two strategies (55). Twenty patients underwent immediate hybrid ablation, while 40 patients underwent a staged procedure. After a mean follow-up of 74 months, no significant difference was noted between the two groups in the risk of AF recurrence [immediate 1/20 (5%) vs. staged 7/40 (17.5%); P=0.18].
Additionally, a designated blanking period is reported to be helpful between surgical and the transcatheter stages. This is important in order to allow the ablation lines to fully stabilize irreversibly. Then, any gaps can be easily recognized and completed by means of endocardial touch-ups. It should be noted that additional peri-mitral and tricuspid lines (mitral and tricuspid isthmuses) as well as the coronary sinus ablation are only required in patients experiencing left or right atrial flutter during blanking period or in patients in whom this supraventricular arrhythmia can be induced during CA. Of note, it seems that only 10% to 15% of patients are at risk for this complication, thus, those lines should be left to the CA stage if needed (38,56).
The safety and the effectiveness profile of the “hybrid convergent” ablation was evaluated in a recent meta-analysis (44). Ablation device improvement and ongoing learning curve have reduced the number of serious complications from 9.0%, pooled from a previous meta-analysis, to 6% (95% CI: 3–8%). In patients with drug-refractory persistent and long-standing persistent AF, freedom from AF was 69% (95% CI: 61–78%) and freedom from AF without AAD was 50% (95% CI: 42–58%) at 1 year follow-up (57).
The lower rhythm outcomes can be attributed to the fact that patients referred for convergent ablation are at very high risk of recurrence due to longer AF duration. However, in these patients, LAA was not managed. Only one randomized controlled study on newer devices was currently published where patients were randomized to either convergent hybrid or CA alone (58).
At 12 months, freedom from atrial tachycardias was 67.7% (67/99) in the hybrid group and 50.0% (25/50) in the CA group (P=0.036), while freedom from atrial tachycardia without AAD was 53.5% (53/99) vs. 32.0% (16/50; P=0.0128), respectively. Evaluation through 7-day Holter at 18 months showed ≥90% AF burden reduction in 74% (53/72) of patients in the hybrid group and 55% (23/42) of patients in the CA group. This aspect is extremely important particularly when referred to non-paroxysmal patients. The Heart Rhythm Society (HRS) consensus statement (59) promoted the global AF burden reduction as an important endpoint after AF ablations, either surgical, catheter or hybrid being associated with a significant reduction in the incidence of cardiac death, cerebrovascular events and heart failure. Accordingly, a recent single center experience showed a global reduction of the AF burden of 90% in non-PAF patients at 7-year of follow-up (31). This result confirmed the global AF burden reduction reported in the HARTCAP-AF trial (50).
Conclusions
The armamentarium for hybrid AF ablation consists of different surgical techniques that can be effectively combined to CA in order to tackle a complex AF substrate as occurs in patients with persistent and long-standing persistent AF. Hybrid AF ablation utilizes the combined strengths of electrophysiology and ablation surgery to offer improved rhythm outcomes when compared to CA alone. Further technical improvements are warranted in order to perform hybrid procedures perfectly reproducing lines from the gold standard Cox-Maze IV.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: C.M. discloses financial relationship with Corcym, Atricure, Estech, Allergan. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kornej J, Börschel CS, Benjamin EJ, et al. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ Res 2020;127:4-20. [Crossref] [PubMed]
- Zulkifly H, Lip GYH, Lane DA. Epidemiology of atrial fibrillation. Int J Clin Pract 2018;72:e13070. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. Erratum in: Eur Heart J 2021;42:507; Eur Heart J 2021;42:546-7; Eur Heart J 2021;42:4194. [Crossref] [PubMed]
- Hassan SM, Hong K, Rosati F, et al. Hybrid ablation for atrial fibrillation: the importance of achieving transmurality and lesion validation. Minerva Cardioangiol 2019;67:115-20. [Crossref] [PubMed]
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83.
- Damiano RJ Jr, Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 2011;141:113-21. [Crossref] [PubMed]
- Gammie JS, Haddad M, Milford-Beland S, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg 2008;85:909-14. [Crossref] [PubMed]
- Santangeli P, Zado ES, Hutchinson MD, et al. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm 2016;13:374-82. [Crossref] [PubMed]
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812-22. [Crossref] [PubMed]
- Hummel J, Michaud G, Hoyt R, et al. Phased RF ablation in persistent atrial fibrillation. Heart Rhythm 2014;11:202-9. [Crossref] [PubMed]
- Clarnette JA, Brooks AG, Mahajan R, et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace 2018;20:f366-76. [Crossref] [PubMed]
- Scherr D, Khairy P, Miyazaki S, et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18-24. [Crossref] [PubMed]
- de Groot N, van der Does L, Yaksh A, et al. Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans. Circ Arrhythm Electrophysiol 2016;9:e003648. [Crossref] [PubMed]
- Parameswaran R, Kalman JM, Royse A, et al. Endocardial-Epicardial Phase Mapping of Prolonged Persistent Atrial Fibrillation Recordings: High Prevalence of Dissociated Activation Patterns. Circ Arrhythm Electrophysiol 2020;13:e008512. [Crossref] [PubMed]
- Rosati F, Hassan SMA, Reid K, et al. Left atrium perforation with lung injury after catheter ablation. J Card Surg 2020;35:2860-2. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J Am Coll Cardiol 2016;68:1929-40. [Crossref] [PubMed]
- De Potter T, Van Herendael H, Balasubramaniam R, et al. Safety and long-term effectiveness of paroxysmal atrial fibrillation ablation with a contact force-sensing catheter: real-world experience from a prospective, multicentre observational cohort registry. Europace 2018;20:f410-8. [Crossref] [PubMed]
- Whitaker J, Fish J, Harrison J, et al. Lesion Index-Guided Ablation Facilitates Continuous, Transmural, and Durable Lesions in a Porcine Recovery Model. Circ Arrhythm Electrophysiol 2018;11:e005892. [Crossref] [PubMed]
- Calzolari V, De Mattia L, Indiani S, et al. In Vitro Validation of the Lesion Size Index to Predict Lesion Width and Depth After Irrigated Radiofrequency Ablation in a Porcine Model. JACC Clin Electrophysiol 2017;3:1126-35. [Crossref] [PubMed]
- Phlips T, Taghji P, El Haddad M, et al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace 2018;20:f419-27. [Crossref] [PubMed]
- Wittkampf FHM, van Es R, Neven K. Electroporation and its Relevance for Cardiac Catheter Ablation. JACC Clin Electrophysiol 2018;4:977-86. [Crossref] [PubMed]
- Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822-8. [Crossref] [PubMed]
- Lim HS, Hocini M, Dubois R, et al. Complexity and Distribution of Drivers in Relation to Duration of Persistent Atrial Fibrillation. J Am Coll Cardiol 2017;69:1257-69. [Crossref] [PubMed]
- Santucci PA, Varma N, Cytron J, et al. Electroanatomic mapping of postpacing intervals clarifies the complete active circuit and variants in atrial flutter. Heart Rhythm 2009;6:1586-95. [Crossref] [PubMed]
- Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101:406-26.
- Nabar A, Rodriguez LM, Timmermans C, et al. Effect of right atrial isthmus ablation on the occurrence of atrial fibrillation: observations in four patient groups having type I atrial flutter with or without associated atrial fibrillation. Circulation 1999;99:1441-5. [Crossref] [PubMed]
- Haïssaguerre M, Wright M, Hocini M, et al. The substrate maintaining persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2008;1:2-5. [Crossref] [PubMed]
- Pathik B, Lee G, Sacher F, et al. Epicardial-endocardial breakthrough during stable atrial macroreentry: Evidence from ultra-high-resolution 3-dimensional mapping. Heart Rhythm 2017;14:1200-7. [Crossref] [PubMed]
- Rosati F, Muneretto C, Merati E, et al. Epicardial, Biatrial Ablation With Integrated Uni-bipolar Radiofrequency Technology in Stand-alone Persistent Atrial Fibrillation. Innovations (Phila) 2018;13:114-9. [Crossref] [PubMed]
- Al-Jazairi MIH, Klinkenberg TJ, Van Putte BP, et al. Totally Thoracoscopic Pulmonary Vein Isolation: A Simplified Technique. Innovations (Phila) 2017;12:493-5. [Crossref] [PubMed]
- Muneretto C, Baudo M, Rosati F, et al. Thoracoscopic Surgical Ablation of Lone Atrial Fibrillation: Long-term Outcomes at 7 Years. Ann Thorac Surg 2023;116:1292-9. [Crossref] [PubMed]
- Castellá M, Kotecha D, van Laar C, et al. Thoracoscopic vs. catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. Europace 2019;21:746-53. [Crossref] [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [Crossref] [PubMed]
- Salih M, Darrat Y, Ibrahim AM, et al. Clinical outcomes of adjunctive posterior wall isolation in persistent atrial fibrillation: A meta-analysis. J Cardiovasc Electrophysiol 2020;31:1394-402. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122:109-18. [Crossref] [PubMed]
- Baudo M, Petruccelli RD, D’Alonzo M, et al. Rhythm outcomes of minimally-invasive off-pump surgical versus catheter ablation in atrial fibrillation: A meta-analysis of reconstructed time-to-event data. Int J Cardiol 2023;376:62-75. [Crossref] [PubMed]
- Derval N, Duchateau J, Denis A, et al. Marshall bundle elimination, Pulmonary vein isolation, and Line completion for Anatomical ablation of persistent atrial fibrillation (Marshall-PLAN): Prospective, single-center study. Heart Rhythm 2021;18:529-37. [Crossref] [PubMed]
- Cox JL, Churyla A, Malaisrie SC, et al. A Hybrid Maze Procedure for Long-Standing Persistent Atrial Fibrillation. Ann Thorac Surg 2019;107:610-8. [Crossref] [PubMed]
- Varzaly JA, Lau DH, Chapman D, et al. Hybrid ablation for atrial fibrillation: A systematic review and meta-analysis. JTCVS Open 2021;7:141-54. [Crossref] [PubMed]
- Richardson TD, Shoemaker MB, Whalen SP, et al. Staged versus Simultaneous Thoracoscopic Hybrid Ablation for Persistent Atrial Fibrillation Does Not Affect Time to Recurrence of Atrial Arrhythmia. J Cardiovasc Electrophysiol 2016;27:428-34. [Crossref] [PubMed]
- Bisleri G, Rosati F, Bontempi L, et al. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg 2013;44:919-23. [Crossref] [PubMed]
- Muneretto C, Bisleri G, Rosati F, et al. European prospective multicentre study of hybrid thoracoscopic and transcatheter ablation of persistent atrial fibrillation: the HISTORIC-AF trial. Eur J Cardiothorac Surg 2017;52:740-5. [Crossref] [PubMed]
- Maesen B, La Meir M. Unilateral Left-sided Thoracoscopic Ablation of Atrial Fibrillation. Ann Thorac Surg 2020;110:e63-6. [Crossref] [PubMed]
- Shrestha S, Plasseraud KM, Makati K, et al. Hybrid Convergent ablation for atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm O2 2022;3:396-404. [Crossref] [PubMed]
- Rosati F, de Maat GE, Valente MAE, et al. Surgical clip closure of the left atrial appendage. J Cardiovasc Electrophysiol 2021;32:2865-72. [Crossref] [PubMed]
- Belley-Cote EP, Connolly SJ, Whitlock RP. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;385:1054-5. Reply. [Crossref] [PubMed]
- Tilz RR, Rillig A, Thum AM, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol 2012;60:1921-9. [Crossref] [PubMed]
- Chew DS, Black-Maier E, Loring Z, et al. Diagnosis-to-Ablation Time and Recurrence of Atrial Fibrillation Following Catheter Ablation: A Systematic Review and Meta-Analysis of Observational Studies. Circ Arrhythm Electrophysiol 2020;13:e008128. [Crossref] [PubMed]
- Eranki A, Wilson-Smith AR, Williams ML, et al. Hybrid convergent ablation versus endocardial catheter ablation for atrial fibrillation: a systematic review and meta-analysis of andomized control trials and propensity matched studies. J Cardiothorac Surg 2022;17:181. [Crossref] [PubMed]
- Vroomen M, La Meir M, Maesen B, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation versus transvenous catheter ablation in persistent and longstanding persistent atrial fibrillation (HARTCAP-AF): study protocol for a randomized trial. Trials 2019;20:370. [Crossref] [PubMed]
- Pison L, La Meir M, van Opstal J, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol 2012;60:54-61. [Crossref] [PubMed]
- Maesen B, Pison L, Vroomen M, et al. Three-year follow-up of hybrid ablation for atrial fibrillation. Eur J Cardiothorac Surg 2018;53:i26-32. [Crossref] [PubMed]
- Lockwood D, Nakagawa H, Peyton MD, et al. Linear left atrial lesions in minimally invasive surgical ablation of persistent atrial fibrillation: techniques for assessing conduction block across surgical lesions. Heart Rhythm 2009;6:S50-63. [Crossref] [PubMed]
- Bulava A, Mokracek A, Hanis J, et al. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc 2015;4:e001754. [Crossref] [PubMed]
- Nasso G, Lorusso R, Di Bari N, et al. Hybrid approach for long-standing persistent atrial fibrillation: immediate versus staged treatment. J Cardiothorac Surg 2022;17:274. [Crossref] [PubMed]
- Matsuo S, Wright M, Knecht S, et al. Peri-mitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm 2010;7:2-8. [Crossref] [PubMed]
- Luo X, Li B, Zhang D, et al. Efficacy and safety of the convergent atrial fibrillation procedure: a meta-analysis of observational studies. Interact Cardiovasc Thorac Surg 2019;28:169-76. [Crossref] [PubMed]
- DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ Arrhythm Electrophysiol 2020;13:e009288. [Crossref] [PubMed]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1-160. [Crossref] [PubMed]






