The outcomes of concomitant catheter ablation in non-mitral valve cardiac surgery—a systematic review and meta-analysis of the literature
Introduction
Atrial fibrillation (AF) is the most common form of cardiac arrhythmia, with a critical importance in the perioperative management of cardiac surgery patients. It necessitates prompt management at an appropriate time in a patient’s clinical journey to avoid the devastating sequalae of cardioembolism, and independently, other comorbidities and mortality (1,2). New-onset AF (NOAF) following cardiac surgery affects approximately one-third of patients and is thought to be due to surgically induced trauma, altered hemodynamics, pharmacotherapeutic and physiologic stress, and ischemic time (i.e., myocardial and systemic) (3). However, approximately 10% of patients in the United States present with AF before their index operation, presenting surgeons with an additional clinical conundrum, but the potential for concurrent management (4). Historically, the decision to treat AF concomitantly has been a point of contention, with an absence of evidence dissuading clinicians.
In the contemporary era, good data have illustrated that untreated AF in patients undergoing cardiac operations significantly increases the risk of perioperative stroke, as well as reduces short- and long-term survival. Interventional management with the choice of catheter ablation versus surgical ablation—or a combination of the two as a hybrid approach—has also become a focal point of interest in head-to-head analyses. However, high quality data on the short- and long-term outcomes of patients receiving AF ablation procedures during non-mitral cardiac surgery remains sparse, with previous high-quality analyses limited by small sample sizes and inadequate study powering, leaving uncertainty (5). Clearly delineating outcomes for this patient cohort is critical, given the increasing burden of AF with an ageing population. The present systematic review and meta-analysis will delineate the outcomes of patients undergoing concomitant AF ablation procedures for all-comer, non-mitral valve cardiac surgery.
Methods
Literature search strategy
The methods for this systematic review adhered to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) updated statement (6). Four electronic databases were used to perform the literature searches, encompassing EMBASE, Ovid MEDLINE, PubMed, and SCOPUS. These databases were searched from the date of database inception through to January 31st 2023. For the examination of the outcomes of concomitant AF ablation procedures during non-mitral valve cardiac surgery, a search strategy using the combination of keywords and Medical Subject Headings (MeSH) including (atrial fibrillation OR AF) AND (catheter ablation OR endocardial ablation OR cox maze OR maze) AND (coronary artery bypass surgery OR CABG OR coronary artery grafting OR CAG OR surgical revascularization OR aortic valve surgery OR concomitant) NOT (mitral valve) was utilized and is visually presented by the PRISMA flow diagram (see Figure S1). Predefined selection criteria were applied to assess for inclusion or exclusion (see “Inclusion and exclusion criteria”).
Each study was screened independently by two co-authors (A.R.W.S., C.J.W.S.), with any conflicts resolved prior to progression through mutual agreement. Where the title and/or abstract provided insufficient detail in the determination of relevance for additional screening, a full-text review of the record was carried out in the first instance. The reference lists of included studies were manually assessed to identify any missed papers from the literature search that were also eligible for assessment. In the instance of multiple studies produced by the same author with the same cohort, the most recent paper was included. In the instance where concern regarding duplication existed but was not clear in the manuscript, the authors were contacted to clarify if the cohorts were distinct.
Inclusion and exclusion criteria
Studies were included in the review if: (I) they examined the perioperative and postoperative outcomes of interest in patients undergoing non-mitral valve cardiac surgery with concomitant AF ablation through any means (e.g., surgical ablation, catheter ablation, hybrid ablation, pulmonary vein isolation (PVI), all with/without left atrial reduction surgery) (see “Primary and secondary endpoints”). Series remained eligible for inclusion in the instance of ‘hybrid’ approaches. Left atrial appendage exclusion procedures were assessed for but non-essential. Studies were excluded for: (I) non-English reporting; (II) narrative reports; (III) studies without clear recruiting details; (IV) no mention of perioperative and postoperative patient outcomes; (V) redo procedures with AF ablation; (VI) no specification as to which cardiac procedure was performed; (VII) nil reporting of postoperative outcomes; (VIII) non-adult patients, or those with congenital disorders; (IX) non-isolated coronary artery bypass surgery (i.e., concomitant valve procedures); (X) less than ten patients in their sample sizes. All studies included were elective cohorts. The number of studies excluded for each reason is as follows: (I) non-English reporting: 50; (II) narrative reporting: 95; (III) studies without clear reporting details: 24; (IV) no perioperative and postoperative patient outcomes: 7; (V) redo procedures with AF ablation: 2; (VI) lack of specification regarding which cardiac procedure was performed: 0; (VII) nil reporting of postoperative outcomes: 0; (VIII) non-adult patients/congenital disorders: 40; (IX) non-isolated coronary artery bypass surgery: 50; (X) studies with <10 patients: 0.
Primary and secondary endpoints
The primary endpoints of analysis was freedom from AF following the index operation. Secondary endpoints included baseline demographic data (i.e., age, sex, cross-clamp and cardiopulmonary bypass time, length of intensive care and hospital stay, thirty-day mortality, long-term mortality (as represented through Kaplan-Meier graphs).
Data extraction, critical appraisal, and quality assessment
Two independent reviewers extracted data directly from publication texts, tables, and figures (C.J.W.S., J.S.S.). A third reviewer independently reviewed and confirmed all extracted data (A.R.W.S.). Differing opinions between the two main reviewers were resolved through discussion led by the primary investigator (A.R.W.S.). Attempts were made to clarify insufficient/indistinct data from authors of included studies, as required.
Data was extracted in a way that each study was effectively treated as a case series, irrespective of underlying design. The Canadian Institute of Health Economics Quality Appraisal score was used as the quality assessment tool (7). Studies were defined as low quality with scores ≤11/19, moderate quality 12–14/19, and high quality ≥15/19.
Statistics
A meta-analysis of proportions or means were performed for categorical and continuous variables, as appropriate, by an independent reviewer. A random effects model was used to account for differing regions, surgeon experience, surgical technique and equipment, and management protocols across the included studies. Means and standard deviations were calculated from the median, where reported, using the methods described by Wan and colleagues (8). Pooled data and standard deviations (SD) or standard error (SE) are presented as N (%) ± SD or SE with 95% confidence intervals (CI). For outcome data, heterogeneity amongst studies was assessed using the I2 statistic. Thresholds for these values were considered as low, moderate, and high heterogeneity as 0–49%, 50–75% and greater than or equal to 75%, respectively. Meta-analysis of proportions or means were performed using Stata (version 17.0, StataCorp, TX, USA). Risk of bias was assessed using the “Risk of Bias in Non-randomized Studies-of Interventions” (ROBINS-I) tool and has been visually presented (see Figure S2. Risk of bias assessment) (9). Reporting of individual variables is also noted.
Funnel plots were generated using R [R Core Team (2021)]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R Studio [RStudio Team (2020)] in the R Studio environment (RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA, USA), with Egger’s and Begg’s tests applied for assessment of small-study effects and publication bias. Cohorts were sub-grouped based on their index operation, into the following cohorts: (I) those undergoing isolated coronary artery bypass grafting; (II) those undergoing isolated aortic valve replacement; and (III) other procedures or those receiving combined approaches (i.e., coronary grafting with aortic valve replacement, septectomy, etc.). Forest plots of covariates are presented in Figure S3. Funnel plots of study covariates are presented in Figure S4.
Results
Baseline demographic, perioperative, and postoperative data (Table 1)
Table 1
| Surgical cohort by procedure type | Isolated CABG | Isolated CABG (95% CI, I2) |
Isolated AVR | Isolated AVR (95% CI, I2) | CABG + AVR | CABG + AVR (95% CI, I2) |
|---|---|---|---|---|---|---|
| Cohort size | 131.4 (71.2); 9/9 studies |
8.0–270.9, I2=0 | 1,272.3 (1,209) | 1,099–3,643, I2=97% | 330.8 (155.91); 13/13 studies |
25.2–636.4, I2=97% |
| Age (years) | 64.8 (1.4); P<0.001; 9/9 studies |
62.0–67.6, I2=65.5% | 69.8 (1.3); P<0.001; 3/3 studies |
67.2–72.5, I2=13% | 68.2 (1.0); P<0.001; 13/13 studies | 66.2–70.3, I2=98% |
| Comorbidities | ||||||
| Type 2 diabetes mellitus | 54.4 (35.1) | 14.3–123.1, I2=22% | 450.7 (430.7) | 393.5–1, 294.8, I2=80% | 124.2 (39.6) | 20.4–316.9, I2=97% |
| Peripheral vascular disease | 44.0 (16.2) | 11.0–77.01, I2=11% | 12.0 (NE) | 0.1–7.9, I2=92% | 58.3 (22.6) | 11.0–166.1, I2=97% |
| Chronic obstructive pulmonary disease | 37.1 (11.6); 8/9 studies |
14.4–60.0, I2=67% | 4.0 (2.0); 3/3 studies |
53.4 (3.8); 7/13 studies |
16.7–92.6, I2=97% | |
| Cross-clamp time | 86.2 (28.2); P=0.002; 8/9 studies |
30.5–141.9, I2=68% | 100.9 (7.7); P<0.001; 3/3 studies |
85.9–88.9, I2=90% | 100.4 (6.6); P<0.001; 10/13 studies |
87.5–113.4, I2=99% |
| Hospital LOS, days | 7.1 (1.1); P<0.001; 4/9 studies |
4.9–9.3, I2=14% | NE | NE | NE | NE |
| ICU LOS, hours | 55.0 (5.38); P<0.001; 2/9 studies |
45.2–66.3, I2=68 | NE | NE | NE | NE |
Data are presented as mean (SE) unless otherwise stated. CABG, coronary artery bypass grafting surgery; CI, confidence interval; AVR, aortic valve replacement; ICU, intensive care unit; LOS, length of stay; NE, non-executable due to insufficient data/imputation limits; SE, standard error.
On application of the search terms, a total of 3,707 studies were identified. Following use of the inclusion and exclusion criteria, 22 studies were identified for inclusion, with detailed characteristics provided in Table S1. Detailed study characteristics (4,10-30). One paper published key clinical data in an earlier study of the same cohort, and hence both papers were included; the patient cohort demographics and outcome data are only counted on a single instance-quality and risk of bias assessment were carried out separately to ensure consistent study quality (14,15). Detailed demographic and procedural information for the included studies are reported in Table S2. Detailed demographics. A total of 9,482 patients were identified, of which 67% were male (6,338/9,482). Isolated coronary artery bypass grafting was the main procedure completed (9/21). The remaining procedures were a combination of coronary artery bypass grafting with concomitant aortic valve repair, and other non-mitral procedures (i.e., septal myectomy) (9/21), followed by isolated aortic valve replacement (3/21). Seven of the studies were from United States centers, ten studies were from European centers, and the remainder were drawn from Asian centers. Nine of the studies were prospective in design, with the remainder as retrospective. Fourteen studies utilized the Cox-Maze procedure (with or without PVI and left atrial appendage exclusion). The remainder utilized PVI with or without left atrial appendage exclusion. The majority of studies followed patients across a three-monthly follow-up period until one year, and then shifted to an annual schedule thereafter. A variety of monitoring of AF freedom was utilized; predominantly, centers utilized echocardiogram with or without longer Holter monitoring. Echocardiography was only utilized at follow-up in two studies. Where reported, there was an even distribution of patients presenting with paroxysmal AF and persistent AF who underwent AF ablation (732 vs. 767, respectively). The majority of patients had mild left ventricular dysfunction. The majority of patients had at least mild to moderate left atrial enlargement.
Begg’s and Egger’s test of small-study effects and publication bias
On assessment of small-study effects and publication bias for the entire cohort’s freedom from AF, a moderate positive correlation between effect size and study precision was illustrated and was statistically significant. Egger’s test for funnel plot asymmetry demonstrated decent evidence of asymmetry, which was also statistically significant. Kendall’s tau =0.55 (P=0.0041), z=4.6 (P≤0.0001), limit estimate b=0.38 (95% CI: 0.038–0.72) (see Figures S5,S6).
Isolated coronary artery bypass grafting cohort
The mean cohort size of the isolated coronary bypass group was 131.4 patients, with a mean age of 64.8 years. Forty-one percent of patients in this cohort had comorbid diabetes, 33% had peripheral vascular disease, and 28% had chronic obstructive pulmonary disease. The mean cross-clamp time for this cohort was 86.2 minutes. The mean hospital length of stay was 7.1 days and had an average of 55.0 hours in the intensive care unit (ICU).
Isolated aortic valve replacement cohort
The mean cohort size of the isolated aortic valve replacement was 1,273 patients, with a mean age of 69.8 years. Thirty-five percent of patients had comorbid diabetes. The mean cross-clamp time was 100.9 minutes. Insufficient data were present to determine the mean hospital or ICU length of stay.
Combined procedure cohort
The mean cohort size of this cohort was 330.8 patients, with a mean age of 68.2 years. Thirty-seven percent of patients had comorbid diabetes, 18% had peripheral vascular disease, and 16% had chronic obstructive pulmonary disease. The mean cross-clamp time was 100.4 minutes. Insufficient data were present to determine the mean hospital or ICU length of stay.
Quality and risk of bias assessment
On quality assessment, 8 studies were deemed to be of high quality (10,11,14,17-19,23,24), 12 were deemed of moderate quality (4,12,13,15,16,20,21,25-28), and 2 were deemed of low quality (29,30). All studies were deemed to be of low risk of bias, with no study being assessed as having more than two domains at moderate risk of concern for bias.
Kaplan-Meier assessment of freedom from AF and mortality
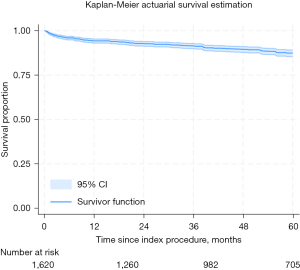
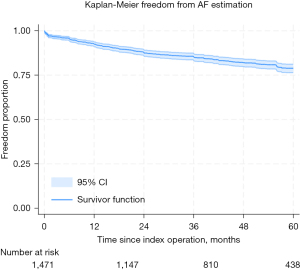
On actuarial assessment, freedom from AF was found to be 93%, 88%, 85%, 82%, and 79% at 1 through to 5 years, respectively (see Figure 1. All-comer freedom from AF from index procedure). Freedom from mortality was found to be 94%, 93%, 91%, 90%, and 87% at 1 through to 5 years, respectively (see Figure 2. All-comer freedom from mortality from index procedure).
Discussion
Untreated AF has a significant association with poor prognostic outcomes in the perioperative setting of cardiac surgery (14,31-33). With an increasingly aging population, the burden of AF in those presenting for their index operation is likely to increase significantly in coming years from the current estimation of 10% of patients. Emerging evidence from randomized trials has demonstrated that ablation is both efficacious and safe, particularly in those undergoing mitral valve operations (34). Data on those undergoing concomitant AF ablation in non-mitral cardiac procedures is lacking, however, and debate still remains as to whether a combined approach should be undertaken (35). The present systematic review and meta-analysis set out to define the long-term outcomes of patients undergoing concomitant AF ablation procedures with non-mitral cardiac surgery and found encouraging results at a maximal follow-up period of 5 years.
Historically, the debate surrounding concomitant therapy centered on a number of factors, principally: (I) surgeon perception that the benefits of restoration of sinus rhythm did not outweigh the additional operative complexity of forming lesion sets, with prolongation of cross-clamp, pump times, etc.; (II) insufficient data on long-term freedom from AF in concomitant cohorts; and (III) a critical difference in the operative approach in the non-mitral setting as additional/seemingly non-essential incisions would have to be made to enter the left atrium, that being, in those where left atriotomy is not already taking place, is there a benefit to doing so purely for ablation (11)? The 2012 Heart Rhythm Society (HRS), the European Heart Rhythm Association (EHRA), and the European Cardiac Arrhythmia Society (ECAS) Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation recommended that on the basis of emerging clinical experience, that it was appropriate for surgeons to consider ablation to increase the incidence of sinus rhythm at short- and long-term follow-up, yet an absence of uptake persists (36).
The results of the present meta-analysis demonstrate a freedom from AF of 79% at a maximal follow-up period of 5 years, superior to that of previously identified rates in earlier meta-analyses in the 2010s (5,37). It is probable that the most efficacious management of AF in the perioperative setting will involve a combination of interventions, including the formation of lesion sets in a bi-atrial manner (as appropriate), PVI, and exclusion of the appendage as an addendum to mitigate further cardioembolic risk, though appendage exclusion is currently a class II recommendation. In the present systematic review, Cox-Maze lesions as stand-alone ablation procedures predominated, but only marginally, with either a combination of Cox-Maze and PVI or PVI as a stand-alone procedure completed in nine of the remaining studies, reflecting this multimodal sentiment. Although the Cox-Maze procedure remains the gold standard for surgical management of AF, it is unlikely that all patients will retain the necessary risk-benefit ratio of undergoing the approach, however (11). The type of AF is critical in determining whether a single, less aggressive modality of ablation (e.g., PVI alone) would be sufficient, with paroxysmal AF far more likely to be terminated than permanent-persistent AF (38). The counterpoint with more aggressive lesion sets is the well-documented likelihood of readmission for heart block, congestive heart failure, pleural effusion, and the need for permanent pacemaker implantation (39). Cardioembolic events are significantly reduced in comparison to cardiac surgery alone cohorts, however (16). The safety of surgical ablation, encouragingly, also appears to be preserved with age (40).
Additional consideration to left atrial dimensions should also be given, as those with greater dilatation and surface area are more likely to have persistence of AF to such a degree where further therapies—both medical and interventional—are required (41). It has been suggested that in those where left atrial dimensions exceed 55 mm, an aggressive atrial reduction approach should take place to provide effective levels of AF treatment (42). With respect to minimally invasive and beating-heart cohorts, Balasubramanian et al. reported on their cohort of patients undergoing off-pump, total arterial revascularization with complete epicardial pulmonary vein encircling, with persistence of sinus rhythm in 71% of patients out to 6 months; Maessen et al. reported superior results in an earlier study at 86% success, though their cohort was smaller, and had continuation of anti-arrhythmic therapy thereafter (13,43). It would appear to be a clear aim, as surgical interventionalists, to keep patients off of anti-arrhythmic therapy, given the additional burden of adverse effects associated with medications such as amiodarone.
van der Heijden et al. report both safety and efficacy in their cohort of patients undergoing minimally invasive coronary bypassing with thoracoscopic harvesting, with satisfactory results at 12 months, though clearly the complexity of these procedures would be even greater than in open approaches (28). Very few studies on minimally invasive cardiac surgery with concomitant AF ablation exist, likely reflecting this difficulty (44). The role of catheter ablation as another potential minimally invasive option, also remains debatable. The principal concern is that the rates of sinus rhythm maintenance is significantly variable across existing long-term studies, with some groups reporting rates of freedom from AF in the 70%s, versus only offering 50% freedom or less in others (45,46); it seems likely that this variability pertains to the difficulty of achieving a transmural outcome through this approach. In light of this, it would seem definitive surgical management at point of operation should be the standard of care.
Limitations
There are a number of limitations inherent to the present review. Firstly, this review did not further subgroup studies that completed atrial exclusion in isolation from procedures that did or partition those cohorts that underwent PVI in isolation versus PVI + Cox-Maze, given that the data would be excessively diluted. This does introduce a moderate degree of heterogeneity. With respect to variable reporting, noticeably, mean operation time was poorly reported; this is presumably as cross-clamp time is the more important variate of the two to consider (with respect to the actual effects of the operation being prolonged by an ablation procedure).The heterogeneity of the data is an additional limitation, likely existent due to a combination of variable ablation lines, levels of surgical experience, differing means of patient selection/enrolment, energy sources, and degrees of paroxysmal vs. persistent AF and left atrial dimensions.
Loss to follow-up was not as major of a concern, though attrition of patient numbers across time will have a degree of influence over the data (i.e., survivor bias, over-estimation of degrees of freedom, etc.). Intrinsic to the limitations of systematic reviews and meta-analyses, the follow-up monitoring methods also varied, though the majority of studies employed ECGs with or without extended Holter monitoring. Additionally, actuarial assessment of cardioembolic risk, though already largely solidified, would be of benefit to aggregate and should be a focus of future studies. Stratification by energy source and/or the extent of the ablation undertaken was also not possible given limited reporting.
Conclusions
This review demonstrated excellent freedom from AF out to a long-term follow-up of 5 years in a robust clinical cohort, with improvements over previous publications. Freedom from mortality was also encouraging at 5 years. Emerging data are increasingly illustrating that in this patient cohort, concurrent treatment of pre-existent AF with cardiac and/or valvular disease at the point of operation should be the standard of care. Key data in the form of adequately powered, randomized control trials will help solidify this assertion.
Acknowledgments
Funding: This research is supported by
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Longstreth WT Jr, Bernick C, Fitzpatrick A, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology 2001;56:368-75. [Crossref] [PubMed]
- van Diepen S, Bakal JA, McAlister FA, et al. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation 2011;124:289-96. [Crossref] [PubMed]
- McIntyre WF. Post-operative atrial fibrillation after cardiac surgery: Challenges throughout the patient journey. Front Cardiovasc Med 2023;10:1156626. [Crossref] [PubMed]
- Al-Atassi T, Kimmaliardjuk DM, Dagenais C, et al. Should We Ablate Atrial Fibrillation During Coronary Artery Bypass Grafting and Aortic Valve Replacement? Ann Thorac Surg 2017;104:515-22. [Crossref] [PubMed]
- Phan K, Xie A, La Meir M, et al. Surgical ablation for treatment of atrial fibrillation in cardiac surgery: a cumulative meta-analysis of randomised controlled trials. Heart 2014;100:722-30. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Institute of Health Economics. Quality Appraisal of Case Series Study Tool. Institute of Health Economics, Edmonton, 2016.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Ad N, Holmes SD, Rongione AJ, et al. The long-term safety and efficacy of concomitant Cox maze procedures for atrial fibrillation in patients without mitral valve disease. J Thorac Cardiovasc Surg 2019;157:1505-14. [Crossref] [PubMed]
- Akpinar B, Sanisoglu I, Guden M, et al. Combined off-pump coronary artery bypass grafting surgery and ablative therapy for atrial fibrillation: early and mid-term results. Ann Thorac Surg 2006;81:1332-7. [Crossref] [PubMed]
- Bakir NH, MacGregor RM, Khiabani AJ, et al. Concomitant Cox-Maze IV and Septal Myectomy in Patients With Hypertrophic Obstructive Cardiomyopathy. Ann Thorac Surg 2022;113:109-17. [Crossref] [PubMed]
- Balasubramanian SK, Theologou T, Birdi I. Microwave surgical ablation for atrial fibrillation during off-pump coronary artery surgery using total arterial-Y-grafts: an early experience. Interact Cardiovasc Thorac Surg 2007;6:447-50. [Crossref] [PubMed]
- Cherniavsky A, Kareva Y, Pak I, et al. Assessment of results of surgical treatment for persistent atrial fibrillation during coronary artery bypass grafting using implantable loop recorders. Interact Cardiovasc Thorac Surg 2014;18:727-31. [Crossref] [PubMed]
- Chernyavskiy A, Kareva Y, Pak I, et al. Quality of Life after Surgical Ablation of Persistent Atrial Fibrillation: A Prospective Evaluation. Heart Lung Circ 2016;25:378-83. [Crossref] [PubMed]
- Churyla A, Andrei AC, Kruse J, et al. Safety of Atrial Fibrillation Ablation With Isolated Surgical Aortic Valve Replacement. Ann Thorac Surg 2021;111:809-17. [Crossref] [PubMed]
- Henn MC, Lawrance CP, Sinn LA, et al. Effectiveness of Surgical Ablation in Patients With Atrial Fibrillation and Aortic Valve Disease. Ann Thorac Surg 2015;100:1253-9; discussion 1259-60. [Crossref] [PubMed]
- Kainuma S, Mitsuno M, Toda K, et al. Dilated left atrium as a predictor of late outcome after pulmonary vein isolation concomitant with aortic valve replacement and/or coronary artery bypass grafting†. Eur J Cardiothorac Surg 2015;48:765-77; discussion 777. [Crossref] [PubMed]
- Kainuma S, Mitsuno M, Toda K, et al. Surgical Ablation Concomitant With Nonmitral Valve Surgery for Persistent Atrial Fibrillation. Ann Thorac Surg 2021;112:1909-20. [Crossref] [PubMed]
- Khargi K, Lemke B, Deneke T. Concomitant anti-arrhythmic procedures to treat permanent atrial fibrillation in CABG and AVR patients are as effective as in mitral valve patients. Eur J Cardiothorac Surg 2005;27:841-6. [Crossref] [PubMed]
- Khargi K, Lemke B, Haardt H, et al. Concomitant anti-arrhythmic surgery, using irrigated cooled-tip radiofrequency ablation, to treat permanent atrial fibrillation in CABG patients: expansion of the indication? Eur J Cardiothorac Surg 2004;25:1018-24. [Crossref] [PubMed]
- Miyairi T, Miura S, Kigawa I, et al. Mid-term results of a closed biatrial procedure using bipolar radiofrequency ablation concomitantly performed with non-mitral cardiac operations. Interact Cardiovasc Thorac Surg 2009;9:169-72. [Crossref] [PubMed]
- Pokushalov E, Romanov A, Cherniavsky A, et al. Ablation of paroxysmal atrial fibrillation during coronary artery bypass grafting: 12 months' follow-up through implantable loop recorder. Eur J Cardiothorac Surg 2011;40:405-11. [Crossref] [PubMed]
- Pokushalov E, Romanov A, Corbucci G, et al. Benefit of ablation of first diagnosed paroxysmal atrial fibrillation during coronary artery bypass grafting: a pilot study. Eur J Cardiothorac Surg 2012;41:556-60. [Crossref] [PubMed]
- Rankin JS, Lerner DJ, Braid-Forbes MJ, et al. Surgical ablation of atrial fibrillation concomitant to coronary-artery bypass grafting provides cost-effective mortality reduction. J Thorac Cardiovasc Surg 2020;160:675-686.e13. [Crossref] [PubMed]
- Suwalski P, Kowalewski M, Jasiński M, et al. Surgical ablation for atrial fibrillation during isolated coronary artery bypass surgery. Eur J Cardiothorac Surg 2020;57:691-700. [Crossref] [PubMed]
- Takai H, Miyata H, Motomura N, et al. Comparison of early outcomes of surgical ablation procedures for atrial fibrillation concomitant to non-mitral cardiac surgery: a Japan Adult Cardiovascular Surgery Database study. Gen Thorac Cardiovasc Surg 2017;65:500-5. [Crossref] [PubMed]
- van der Heijden CAJ, Segers P, Masud A, et al. Unilateral left-sided thoracoscopic ablation of atrial fibrillation concomitant to minimally invasive bypass grafting of the left anterior descending artery. Eur J Cardiothorac Surg 2022;62:ezac409. [Crossref] [PubMed]
- Yildirim Y, Petersen J, Aydin A, et al. Complete Left-Atrial Lesion Set versus Pulmonary Vein Isolation Only in Patients with Paroxysmal AF Undergoing CABG or AVR. Medicina (Kaunas) 2022;58:1607. [Crossref] [PubMed]
- Yoo JS, Kim JB, Ro SK, et al. Impact of concomitant surgical atrial fibrillation ablation in patients undergoing aortic valve replacement. Circ J 2014;78:1364-71. [Crossref] [PubMed]
- Attaran S, Saleh HZ, Shaw M, et al. Does the outcome improve after radiofrequency ablation for atrial fibrillation in patients undergoing cardiac surgery? A propensity-matched comparison. Eur J Cardiothorac Surg 2012;41:806-10; discussion 810-1. [Crossref] [PubMed]
- Ngaage DL, Schaff HV, Barnes SA, et al. Prognostic implications of preoperative atrial fibrillation in patients undergoing aortic valve replacement: is there an argument for concomitant arrhythmia surgery? Ann Thorac Surg 2006;82:1392-9. [Crossref] [PubMed]
- Saxena A, Dinh D, Dimitriou J, et al. Preoperative atrial fibrillation is an independent risk factor for mid-term mortality after concomitant aortic valve replacement and coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg 2013;16:488-94. [Crossref] [PubMed]
- Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399-409. [Crossref] [PubMed]
- La Meir M, Gelsomino S, Nonneman B. The problem with concomitant atrial fibrillation in non-mitral valve surgery. Ann Cardiothorac Surg 2014;3:124-9. [Crossref] [PubMed]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528-606. [Crossref] [PubMed]
- Cheng DC, Ad N, Martin J, et al. Surgical ablation for atrial fibrillation in cardiac surgery: a meta-analysis and systematic review. Innovations (Phila) 2010;5:84-96. [Crossref] [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [Crossref] [PubMed]
- Churyla A, Karim A, Andrei AC, et al. New pacemaker requirements after surgical ablation of atrial fibrillation during valve surgery. J Am Coll Cardiol 2019;73:532.
- Petersen J, Vettorazzi E, Hakmi S, et al. Should concomitant surgical ablation for atrial fibrillation be performed in elderly patients? J Thorac Cardiovasc Surg 2021;161:1816-1823.e1. [Crossref] [PubMed]
- Pecha S, Hakmi S, Subbotina I, et al. Concomitant surgical ablation for atrial fibrillation (AF) in patients with significant atrial dilation >55 mm. Worth the effort?. J Cardiothorac Surg. 2015;10:165. [Crossref] [PubMed]
- Badhwar V, Rovin JD, Davenport G, et al. Left atrial reduction enhances outcomes of modified maze procedure for permanent atrial fibrillation during concomitant mitral surgery. Ann Thorac Surg 2006;82:1758-63; discussion 1764. [Crossref] [PubMed]
- Maessen JG, Nijs JF, Smeets JL, et al. Beating-heart surgical treatment of atrial fibrillation with microwave ablation. Ann Thorac Surg 2002;74:S1307-11. [Crossref] [PubMed]
- Phan K, Croce B, Yan TD. Minimally invasive atrial fibrillation surgery. Ann Cardiothorac Surg 2014;3:130. [Crossref] [PubMed]
- Tzou WS, Marchlinski FE, Zado ES, et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:237-42. [Crossref] [PubMed]
- Ngo L, Lee XW, Elwashahy M, et al. Freedom from atrial arrhythmia and other clinical outcomes at 5 years and beyond after catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes 2023;9:447-58. [Crossref] [PubMed]