Atrial fibrillation ablation during robotic mitral valve surgery: a systematic review and meta-analysis
Introduction
Atrial fibrillation (AF) is a common tachyarrhythmia, affecting approximately 30 million people worldwide (1,2). Mitral valve disease, in particular, has a strong association with AF, with 30–40% of patients developing AF (3). The Cox-Maze IV procedure remains the gold standard for the surgical management of AF (4). This procedure initially involved making a series of incisions on the atria and subsequently suturing the lines, thereby interrupting the micro re-entrant circuits responsible for AF (5). With the advent of alternative energy sources such as cryothermia and/or radiofrequency, the same lesion set can be made with greater ease, providing the basis for modern iterations of the Cox-Maze IV procedure. The Cox-Maze IV procedure has excellent efficacy, with a 5- and 10-year freedom from atrial tachyarrhythmia of 84% and 77% respectively (6). The advent of energy sources has also enabled the Cox-Maze IV procedure to be performed through a minimally invasive approach, with encouraging mid-term outcomes (7).
Over the past two decades, minimally invasive approaches for mitral valve surgery have also been utilised (8). Of these, robotic mitral valve surgery, although limited to specialised centres, offers benefits to conventional approaches. Although the benefits of robotic mitral valve surgery are well documented, the literature for combined mitral valve surgery with concomitant AF surgery remains sparse (9,10). A recent meta-analysis by Williams et al. demonstrates a potentially lower mortality and shorter hospital and intensive care unit (ICU) length of stay (10). As robotic mitral valve surgery becomes commonly utilised, there will be an increasing need to perform surgical ablation for AF concomitantly through the same access. This is of increasing importance as guidelines advocate for performing ablation in patients undergoing mitral valve surgery with a history of AF (11,12).
The aim of this systematic review and meta-analysis is to evaluate the evidence assessing the efficacy of AF ablation during robotic mitral valve surgery. The primary outcome is freedom from AF and the secondary aim is to evaluate the safety profile of concomitant ablation.
Methods
Literature search strategy
Six electronic databases were used including Ovid, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews (CDSR) and SCOPUS. These databases were searched from inception to the 14th of April 2023. The search strategy included a combination of keywords and Medical Subject Headings (MeSH), including “Ablation” OR “Maze” OR “Cryomaze” AND “AF” AND “Robotic Mitral Valve” OR “Robotic”. Predefined criteria for selection were used to assess all articles. The article was written in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (13). The PRISMA flowchart is outlined in Figure S1. Two reviewers (D.N. and B.M.) independently screened the abstracts of all identified records. Included titles were then reviewed with a full-text copy by the same two reviewers. Any conflicts were resolved with a third independent reviewer (A.E.). The reference lists of selected studies were manually searched to identify any additional articles.
Selection criteria
Studies were eligible for inclusion if they included a patient population that underwent AF ablation concomitantly with robotic mitral valve surgery. Robotic mitral valve surgery was deemed to be any operation involving a robotic console on the mitral valve, whereby the mitral disease was primary pathology. AF ablation was defined as any cut/sew lines, radiofrequency, or cryoablation performed on the heart (i.e., either epicardial or endocardial). The inclusion criteria were: (I) AF ablation concurrently with mitral valve surgery; (II) mitral valve surgery as the primary pathology and indication for surgery; (III) operation performed robotically; (IV) freedom from AF reported for the cohort of patients undergoing robotic mitral valve surgery; and (V) published after 2000. Studies which reported concomitant aortic valve surgery and coronary artery bypass grafting (CABG) were included as long as mitral valve surgery was the primary indication. Studies that had mixed populations that did not delineate between pathologies were excluded. Studies which performed mitral valve surgery through a sternotomy were excluded. When institutions published duplicate studies with extended length of follow-up or larger study populations, the most updated and complete study was included. Abstracts, case reports, conference presentations, editorials, and reviews were excluded.
Outcomes
The primary outcome was defined as freedom from AF, as defined by the study. Freedom from AF at both reported follow-up and at discharge was recorded. Secondary endpoints were short-term mortality (in-hospital or 30-day mortality), postoperative stroke, conversion to sternotomy and long-term pacemaker insertion.
Data extraction and statistical analysis
Two independent reviewers (A.E. and D.N.) extracted data directly from publication texts, tables, and figures. A third reviewer (B.M.) independently reviewed and confirmed the integrity of all extracted data. Attempts were made to clarify missing data with the authors. For baseline variables, nominal data was recorded as number of events (n) and expressed as a percentage. Continuous variables were either expressed as a mean and standard deviation (SD) or median and interquartile range (IQR). For statistical analysis, medians and IQR were converted to mean and SD utilising the method outlined by Wan et al. (14). Statistical analysis was carried out using Stata® (Version 17.0, StataCorp, Texas, USA). Baseline continuous data was collated using the metan function and the pooled result was expressed as a weighted mean (n) and 95% confidence interval (CI). To summarize outcome data, a meta-analysis of proportions was performed using the metaprop function, with a Freeman-Tukey arcsine transformation. A random effects model was utilized to account for varied study design, year of publication, lesion sets, center protocol, and population. Results were expressed as forest plots where appropriate, with cumulative proportion expressed as a single percentage. Heterogeneity was assessed using the I2 test statistic. Low heterogeneity was denoted by I2<50%, moderate heterogeneity by I2=50–74%, and high heterogeneity by I2≥75%. Statistical significance was denoted by P<0.05. Study quality was assessed utilising the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool, scoring studies as “Low”, “Moderate” or “Serious” risk of bias.
Results
Our search produced a total of 580 articles, of which 165 duplicates were removed. Four hundred and fifteen abstracts were screened, and 14 were selected for full-text review. After excluding 9 studies, 5 studies were selected for inclusion (15-19). All studies were cohort studies, of which two were prospective and two were retrospective. One study did not delineate whether the study design was retrospective or prospective (19). The patient cohort was largely patients undergoing robotic mitral valve surgery and concomitant AF ablation. One study included all patients undergoing robotic mitral valve surgery and included a subset of patients undergoing concomitant AF ablation (18). The total number of patients was small, ranging from 11 to 94. The aggregate mean reported follow-up was 6.9 months (95% CI: 4.8–9.0). ECG was the main method of monitoring and three studies utilised continuous monitoring such as a Holter. Study level data is summarised in Table 1.
Table 1
| Primary author | Study period | Country | Study design | Patient cohort | Total patients | Mean follow-up time (months) | Reported follow-up time (months) | Method of monitoring |
|---|---|---|---|---|---|---|---|---|
| Aydin et al. | 2013–2018 | Turkey | Retrospective | Robotic MV surgery and AF ablation | 11 | 16.3±5.42 | 16.3±5.42 | ECG |
| Ju et al. | 2007–2017 | South Korea | Prospective | Robotic MV surgery and AF ablation | 94 | 32.1±9.19 | 3.0±0 | ECG and Holter |
| Kadan et al. | 2014–2020 | Turkey | Retrospective | Robotic MV surgery and AF cryoablation | 34 | 23.3±19.4 | 23.3±19.4 | Holter, ECG and TTE |
| Nifong et al. | 2000–2010 | USA | Prospective | Robotic MV surgery | 86 | 1.0±0.8 | 1.0±0.8 | ECG |
| Reade et al. | 2003 | USA | NR | Robotic MV surgery and AF ablation | 16 | 6.0±0 | 6.0±0 | ECG |
MV, mitral valve; AF, atrial fibrillation; ECG, electrocardiogram; TTE, transthoracic echocardiogram; USA, United States of America; NR, not reported.
Demographic data
Demographic data was variably reported. A total of 60.4% of patients were male. The aggregate mean age was 58.5 years (95% CI: 52.6–64.4). Most patients had persistent AF (71.1%). The aggregate mean LVEF was 57.1% (95% CI: 53.8–60.4%). left atrium size was variably reported, with two studies reporting diameter, one reporting left atrial volume index (LAVI) and two not reporting left atrium diameter entirely. This data is summarised in Table 2.
Table 2
| Primary author |
N | Males (n) |
Age (years), mean ± SD |
Paroxysmal AF (%) |
Persistent AF (%) |
LVEF (%), mean ± SD |
LA diameter (mm), mean ± SD |
|---|---|---|---|---|---|---|---|
| Aydin et al. | 11 | 7 | 45.8±16.6 | 0 | 100 | 55.2±7.9 | 69.6±4.9 mL/m2 (LAVI) |
| Ju et al. | 94 | 67 | 53.9±12.7 | 23.4 | 76.6 | 60.3±8.0 | 55.0±8.4 |
| Kadan et al. | 34 | 10 | 58.1±9.8 | 2.9 | 97.1 | 55.0±11.9 | 64.2±10.7 |
| Nifong et al. | 86 | NR | 65.6±10.8 | 48.8 | 51.2 | NR | NR |
| Reade et al. | 16 | NR | 64.9±8.7 | NR | NR | 56.4±10.2 | NR |
SD, standard deviation; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; LA, left atrium; LAVI, left atrial volume index.
Operative data
All five studies utilised the da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA). All studies performed endocardial lesion sets, with one study producing both epicardial and endocardial lines. The energy source varied, with two studies utilising radiofrequency ablation and three studies using cryoablation. Lesion sets also varied; with three studies performing a left atrial Maze (LAM) and one study performing exclusively a bi-atrial Maze (BAM). One study reported a mixed cohort of LAM and BAM (16). Specific lesion sets also varied and these are represented in Table 3. All studies performed left atrial appendage (LAA) exclusion, and five studies performed this in all patients. The aggregate mean cardiopulmonary bypass time (CPBT) was 186 minutes (95% CI: 162–210), and the corresponding cross clamp time (CCT) was 129 minutes (95% CI: 120–139). The cause of mitral regurgitation (MR) varied significantly and was variably reported between the studies. The majority of patients underwent mitral valve repair with two studies reporting largely mitral valve replacement. The use of antiarrhythmic drugs (AAD) was variable. Three studies uniformly continued AADs postoperatively, while one study did not uniformly utilise AADs, with 9.6% of patient discharged on them overall (16). One study did not report the use of AADs (18). Four studies reported the use of anticoagulation, and continued it in the postoperative phase. Three studies stopped anticoagulation at 3 months if patients were in sinus rhythm, and one study did not specify. Operative data is summarised in Table 3.
Table 3
| Primary author | N | Robot used | MV repair | MV replacement | Rheumatic aetiology | TVR | TVA | ASD closure | Energy source | Lesion set |
LAA exclusion | CPBT (min) |
CCT (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aydin et al. | 11 | Da Vinci | 3/11 | 8/11 | 5 | 2 | 0 | 0 | RF | LAM | 11/11 | 147.9±19.1 | 105.8±20.0 |
| Ju et al. | 94 | Da Vinci | 92/94 | 2/94 | 6 | NR | 34 | 8 | Cryo | BAM 34/94; LAM 60/94 | 18/94 | 222.7±57.8 | 134.1±30.4 |
| Kadan et al. | 34 | Da Vinci | 2/34 | 32/34 | NR | 8 | NR | NR | Cryo | LAM | 34/34 | 196.0±25.6 | 141.8±32.1 |
| Nifong et al. | 86 | Da Vinci | 86/86 | 0/86 | NR | NR | NR | NR | Cryo | BAM | 86/86 | 188.5±53.8 | 130.6±28.4 |
| Reade et al. | 16 | Da Vinci | 16/16 | 0/16 | NR | NR | NR | NR | RF | LAM | 16/16 | 174.0±40.8 | 133.0±30.0 |
MV, mitral valve; TVR, tricuspid valve replacement; TVA, tricuspid valve annuloplasty; ASD, atrial septal defect; LAA, left atrial appendage; CPBT, cardiopulmonary bypass time; CCT, cross-clamp time; RF, radiofrequency; LAM, left atrial Maze; BAM, bi-atrial Maze; Cryo, cryothermy; NR, not reported.
Outcome data and study quality
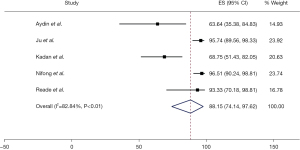
The freedom from AF was 88.1% (95% CI: 74.1–97.6%), at a weighted mean follow-up of 6.9 months (95% CI: 4.8–9.0). This result is associated with significant heterogeneity (I2=83%). Freedom from AF at discharge was 88.0% (95% CI: 72.6–98.1%) as reported by four studies. This result was associated with moderate heterogeneity (I2=74%). These results are summarised in Figures 1,2. Four studies reported no mortality, and one study had two deaths, out of a total of 241 patients (0.8%) (17). Three studies reported postoperative CVA with a total of two cases overall (1.4%). Three studies reported conversion to sternotomy, with a total of two patients requiring this (1.4%). Three studies also reported post-operative permanent pacemaker (PPM) insertion with a total of 8 cases (3.7%). These results are summarised in Table 4. Study quality was variable, with most studies scoring “moderate” in terms of risk of bias. One study was deemed of “low” risk of bias, mainly due to its prospective nature and larger patient cohort (16) (Figure S2).
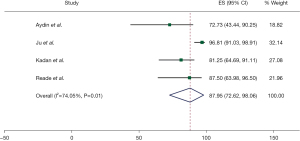
Table 4
| Parameters | Events/total | N | Weighted pooled estimate (%) (95% CI) or proportion (%) | Heterogeneity I2 (%) |
|---|---|---|---|---|
| Freedom from AF | 216/238 | 5 | 88.1 (74.1–97.6) | 82.8 |
| Freedom from AF (at patient discharge) |
139/153 | 5 | 88.0 (72.6–98.1) | 74 |
| Short-term mortality | 2/241 | 5 | 0.8 | NA |
| CVA (short-term) | 2/139 | 3 | 1.4 | NA |
| Reoperation | 1/139 | 3 | 0.7 | NA |
| Conversion to sternotomy | 2/139 | 3 | 1.4 | NA |
| PPM insertion | 8/214 | 3 | 3.7 | NA |
N, number of studies; CI, confidence interval; AF, atrial fibrillation; CVA, cerebrovascular accident; NA, not available; PPM, permanent pacemaker.
Discussion
AF is the most common cardiac arrhythmia and contributes significantly to cardiovascular mortality and morbidity (20). Traditionally, the cut and sew maze has been effective in managing AF, however, it has not been widely adopted due to the extended time, need for median sternotomy, and the recovery associated with this (7). With the advent of alternative energy sources and flexible tip catheters, AF ablation has evolved to be an endoscopic, off pump and robotically assisted procedure for the management of AF (21). Evidence suggests that these procedures can be performed safely with a high degree of efficacy. Ad et al., performing a BAM as a standalone procedure through a mini-thoracotomy, reported a 5-year sinus rhythm maintenance of 73% (7). The majority of ablations performed in this study were via cryotherapy, utilising a malleable cryothermy device (7). As robotic mitral valve surgery becomes commonly utilised, there will be an increasing need to perform surgical ablation for AF concomitantly through minimally invasive access.
This study reports a pooled freedom from AF of 88.1%, at a weighted mean follow-up of 6.9 months. This result is comparable to, albeit slightly higher than previously published literature (22,23). The freedom from AF at patient discharge was equally high, at 87.9%. There were only two mortalities occurring in one study, out of a total of 241 patients (0.8%). Three studies reported postoperative CVA with a total of two cases (1.4%). Two patients required conversion to sternotomy (1.4%) and eight patients required PPM insertion (3.7%). Even with small patient numbers, these results are consistent with previously published data assessing robotic mitral valve surgery (24). One explanation for this is the exclusion of higher risk patients from robotic mitral valve cohorts, such as those with elevated body mass index (BMI), difficult access, reoperation and pulmonary disease. In addition, all papers within this review had less than 100 patients. The high freedom from AF and low mortality may in part be a sequalae of the selection bias of patients. Furthermore, all papers were non-randomised, adding to potential selection bias. Though the results are promising, further studies with larger cohorts of patients and a randomised design can further elucidate the effectiveness and safety of AF ablation during robotic mitral valve surgery, whilst minimising these biases.
There was significant variation in the freedom from AF between studies and there are a number of reasons for this. The cohort of patients in this systematic review was mainly those with persistent AF. This represents a cohort of patients resistant to ablation, and despite this, achieved a favourable freedom from AF. A degree of variation between the studies can be attributed to this; Kadan et al. (17) operated almost exclusively on a cohort of patients with persistent AF and demonstrated a freedom from AF of 68.8% whereas Nifong et al. (18) had a mixed cohort of paroxysmal and persistent AF, demonstrating a higher freedom from AF of 96.5%. This is in part due to the progressive electro-anatomical changes that occur with AF persistence and the spread of arrhythmogenic triggers and substrates outside of the pulmonary veins (25). As a result, persistent AF is more resistant to ablation (25). Another explanation for the variance was the differing lesion sets between the studies. Nifong et al. was the only study to consistently perform a BAM which may account for the higher freedom from AF encountered in this study (18). Evidence suggests that a BAM has a higher efficacy when compared to LAM, however, this needs to be further elucidated with randomised data (26,27). Issues with right-sided lesions however include increased CPB times, and increased incidence of pacemaker implantation (22). Finally, there was variable use of left atrial volume reduction, with one study performing it on all 11 patients and another performing it on the majority of patients (15,16). Mitral valve disease is associated with increased left atrium size, which is a well-known risk factor for AF recurrence (28). Non-randomised data demonstrates that volume reduction confers a favourable freedom from AF during concomitant ablation and mitral valve surgery. In addition, the cut and sew effect of volume reduction may produce transmural scars, disrupting the re-entrant circuits associated with AF to a greater extent than radiofrequency or cryoablation (29). Though it is technically feasible during robotic mitral valve surgery, the benefits of volume reduction need to be further elucidated with large scale data.
One of the challenges faced by robotic AF ablation in addition to mitral valve surgery is prolonged CCT and CPBT. Robotic surgery alone is associated with longer CCT and CPBT compared to conventional approaches, demonstrated in a recent systematic review by Williams et al. (10). A 2013 systematic review by Seco et al. reported CPBT ranging from 106±22 to 188.5±54 minutes and mean CCT ranging from 79±16 to 140±40 minutes (30). This study reported an aggregate mean CPBT of 186 minutes and CCT of 129 minutes, which was within the realms of data reported for robotic mitral valve surgery alone (30). One included study compared CPBT and CCT between patients undergoing robotic mitral surgery alone vs. robotic mitral valve surgery and AF ablation, demonstrating CPBT of 189 vs. 153 minutes and CCT of 131 and 117 minutes respectively (18). Further evidence is required to compare CPBT and CCT in patients undergoing AF ablation in addition to robotic mitral valve surgery.
The development of flexible tip probes simplifies the creation of ablation lines during robotic surgery. Three studies utilised cryothermy and two utilised radiofrequency as the primary energy source. All studies utilised a long shafted, malleable tip instrument to deliver the energy. The benefits of these include a flexible tip that contours the left and right atrium producing lines and a handle designed for lateral port access (31). In a robot-assisted setting, AF ablation procedures have some technical differences compared with other types of cardiac operations. The ablation device is manipulated by the bedside surgeon, whereas tactile feedback is not received by the console surgeon (16). Proper coordination among surgical team members is needed to make effective contact of the ablation probe (16). Concomitant ablation of AF in a robotic setting is a little more difficult and time consuming compared to standard surgery, and this may serve as a limiting factor in choosing this surgical approach. These challenges can be ameliorated by close cooperation between the console and bedside surgeons. In addition to this, a robotic assisted setting can utilise the camera to provide a close-up endoscopic view of the atria, so that the surgeon can create accurate ablation lines (16).
This systematic review is not without limitations. The most significant limitation is the inclusion of retrospective studies with small patient numbers, ranging from 11 to 94 patients. As a result, it is difficult to draw generalised conclusions from this data. Secondly, this is largely a single arm review, with only one study comparing outcomes of robotic mitral valve surgery to robotic mitral valve surgery with AF ablation. Thirdly, only short-term data has been provided, with a mean follow-up of 6.9 months. As a result, it is difficult to elucidate long term conclusions from this data, especially with respect to sinus rhythm attrition. Fourthly this study represents a highly select group of patients with a number of exclusion criteria, precluding patients from undergoing robotic mitral valve surgery. Future research should be derived from large scale (registry) data reporting efficacy, mortality and morbidity. Following on from this, prospective data in the form of randomised controlled trials may alleviate the selection biases associated with retrospective analysis. Future studies would benefit from comparing the efficacy of various energy sources (cryothermy vs. radiofrequency) in a robotic setting, lesion sets (LAM vs. BAM) and the efficacy of left atrial volume reduction.
Conclusions
AF ablation with robotic mitral valve surgery can be performed with adequate short-term efficacy and safety profile. Current evidence on AF ablation and robotic mitral valve surgery is limited to low-quality retrospective data with inherent selection bias. This meta-analysis demonstrates a freedom from AF of 88.1% at 6.9 months and only two deaths in 241 patients (0.8%). Further high-quality studies in the form of prospectively collected data from randomised controlled trials are required to verify these results and also to compare the effects of lesion sets, energy sources and atrial reduction surgery.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837-47. [Crossref] [PubMed]
- Zoni-Berisso M, Lercari F, Carazza T, et al. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213-20. [Crossref] [PubMed]
- Darby AE, Dimarco JP. Management of atrial fibrillation in patients with structural heart disease. Circulation 2012;125:945-57. [Crossref] [PubMed]
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation: III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83.
- Cox JL, Schuessler RB, Lappas DG, et al. An 8 1/4 -year clinical experience with surgery for atrial fibrillation. Ann Surg 1996;224:267-75. [Crossref] [PubMed]
- Khiabani AJ, MacGregor RM, Bakir NH, et al. The long-term outcomes and durability of the Cox-Maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2022;163:629-641.e7. [Crossref] [PubMed]
- Ad N, Holmes SD, Friehling T. Minimally Invasive Stand-Alone Cox Maze Procedure for Persistent and Long-Standing Persistent Atrial Fibrillation: Perioperative Safety and 5-Year Outcomes. Circ Arrhythm Electrophysiol 2017;10:e005352. [Crossref] [PubMed]
- Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III 1996;319:219-23.
- Cao C, Wolfenden H, Liou K, et al. A meta-analysis of robotic vs. conventional mitral valve surgery. Ann Cardiothorac Surg 2015;4:305-14. [Crossref] [PubMed]
- Williams ML, Hwang B, Huang L, et al. Robotic versus conventional sternotomy mitral valve surgery: a systematic review and meta-analysis. Ann Cardiothorac Surg 2022;11:490-503. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. Erratum in: Eur Heart J 2022;43:2022. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Aydin U, Sen O, Kadirogullari E, et al. Robotic Mitral Valve Surgey Combined with Left Atrial Reduction and Ablation Procedures. Braz J Cardiovasc Surg 2019;34:285-9. [Crossref] [PubMed]
- Ju MH, Huh JH, Lee CH, et al. Robotic-Assisted Surgical Ablation of Atrial Fibrillation Combined With Mitral Valve Surgery. Ann Thorac Surg 2019;107:762-8. [Crossref] [PubMed]
- Kadan M, Kubat E, Erol G, et al. Early- and mid-term results of cryoablation of atrial fibrillation concomitant with robotic mitral valve surgery. Anatol J Cardiol 2021;25:266-72. [Crossref] [PubMed]
- Nifong LW, Rodriguez E, Chitwood WR Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [Crossref] [PubMed]
- Reade CC, Johnson JO, Bolotin G, et al. Combining robotic mitral valve repair and microwave atrial fibrillation ablation: techniques and initial results. Ann Thorac Surg 2005;79:480-4. [Crossref] [PubMed]
- Jeanmart H, Casselman F, Beelen R, et al. Modified maze during endoscopic mitral valve surgery: the OLV Clinic experience. Ann Thorac Surg 2006;82:1765-9. [Crossref] [PubMed]
- Kim JB, Cho WC, Jung SH, et al. Alternative energy sources for surgical treatment of atrial fibrillation in patients undergoing mitral valve surgery: microwave ablation vs cryoablation. J Korean Med Sci 2010;25:1467-72. [Crossref] [PubMed]
- Huffman MD, Karmali KN, Berendsen MA, et al. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. Cochrane Database Syst Rev 2016;2016:CD011814. [Crossref] [PubMed]
- Phan K, Xie A, Tian DH, et al. Systematic review and meta-analysis of surgical ablation for atrial fibrillation during mitral valve surgery. Ann Cardiothorac Surg 2014;3:3-14. [Crossref] [PubMed]
- Williams ML, Eranki A, Mamo A, et al. Systematic review and meta-analysis of mid-term survival, reoperation, and recurrent mitral regurgitation for robotic-assisted mitral valve repair. Ann Cardiothorac Surg 2022;11:553-63. [Crossref] [PubMed]
- Kaba RA, Momin A, Camm J. Persistent Atrial Fibrillation: The Role of Left Atrial Posterior Wall Isolation and Ablation Strategies. J Clin Med 2021;10:3129. [Crossref] [PubMed]
- Gelsomino S, La Meir M, Van Breugel HN, et al. Surgical ablation in patients undergoing mitral valve surgery: impact of lesion set and surgical techniques on long-term success. Europace 2016;18:1528-37. [Crossref] [PubMed]
- Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399-409. [Crossref] [PubMed]
- Scherer M, Therapidis P, Miskovic A, et al. Left atrial size reduction improves the sinus rhythm conversion rate after radiofrequency ablation for continuous atrial fibrillation in patients undergoing concomitant cardiac surgery. Thorac Cardiovasc Surg 2006;54:34-8. [Crossref] [PubMed]
- Kim JH, Jang WS, Kim JB, et al. Impact of volume reduction in giant left atrium during surgical ablation of atrial fibrillation. J Thorac Dis 2019;11:84-92. [Crossref] [PubMed]
- Seco M, Cao C, Modi P, et al. Systematic review of robotic minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:704-16. [Crossref] [PubMed]
- Doll N, Meyer R, Walther T, et al. A new cryoprobe for intraoperative ablation of atrial fibrillation. Ann Thorac Surg 2004;77:1460-2. [Crossref] [PubMed]