How to establish a successful and sustainable surgical atrial fibrillation program: key considerations on the arrhythmia, training and treatment strategies
Even though atrial fibrillation (AF) reflects a serious cardiomyopathy with a significant prevalence in patients undergoing cardiac surgery, the overall approach to treat this arrhythmia concomitantly is still far from what would be recommended by current guidelines (1-4). To build up a solid and successful AF program and to consequently treat AF should not only help a considerable number of patients who otherwise remain exposed to the risk of stroke, hemodynamic compromises, heart failure development and death, but should also reflect today’s standard of care in cardiac surgery.
There are several factors to consider when applying standard of care with regard to surgical ablation, which are essential to leverage an efficacious and sustainable program.
- Awareness;
- Training;
- Standard of treatment;
- Follow-up;
- Interdisciplinary collaboration.
Awareness
Awareness of AF might be one of the most important factors in establishing a successful AF program. The fact that available treatment strategies do not warrant 100% success on the one hand and early recurrences might not necessarily indicate a treatment failure on the other, is a unique situation for a cardiac surgeon. As opposed to other cardiac procedures it is impossible to confirm the result at the time of the procedure or during the hospital stay. This uncertainty in outcome lowers the threshold to ignore treatment options. That is even reinforced by the fact that the potentially harmful impact on the patient by rejecting adequate AF treatment usually occurs much later in the time course and might not be recognized by the surgeon at all or is not considered to be related to the procedure in which AF was ignored.
Current guidelines define AF as an atrial cardiomyopathy. Although some patients experience no symptoms at all, it still increases the risk of stroke by four- to five-fold, leads to heart failure in about 30% of patients, increases mortality by up to three-and-a-half-fold and has also incremental effects on cognitive function and quality of life (2). Therefore, AF has a significant impact and should be considered as a serious heart disease.
Multiple registry data has shown a significant survival benefit in patients who suffer from AF and received ablation therapy at the time of their cardiac surgery compared to those who remain untreated (5,6) (Figure 1).
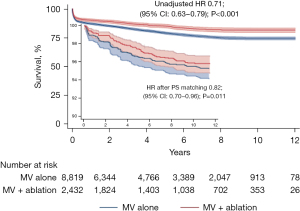
To put the published odd-ratios into perspective: one out of five AF patients could still be alive after five years if AF was not ignored during the performed cardiac procedure. After ten years it is one out of every four patients. Thus, to consider every AF patient for adequate treatment is reflecting today’s standard of care in cardiac surgery and is also recommended by current guidelines.
- AF is a serious cardiac disease with significant mid-term impact on hard endpoints such as mortality, stroke and heart failure. To ignore it and omit treatment does not reflect current standard of care in cardiac surgery.
Training
Training is not limited to the technical aspects of ablation but also includes a thorough understanding of the pathophysiology of AF, the working mechanisms and specifics of different ablation devices and energy sources, as well as the known predictors of therapy failure and potential risk assessment. Moreover, definition of indications as well as a protocol for the treatment strategy might help to standardize the approach to treat AF and thus warrant reproducibility of results.
Pathophysiology of AF from a clinical perspective
As opposed to electrophysiology, cardiac surgery usually lacks the ability to perform epi- or endocardial mapping to understand the current predominant driver of AF. On the other hand, even electrophysiological mapping is only reflective of the status in the current moment and therefore does not necessarily capture all components of a dynamic arrhythmia such as AF.
There is wide consensus that the bi-atrial Maze procedure is the most comprehensive lesion set in surgical ablation and results in the best long-term rhythm outcome, independent of the type of AF (7) (Figure 2). Its different iterations have taught us a lot about the different pathologies of AF and how it is treated most efficaciously.
The Maze principle consists of three factors:
- Isolation of anatomical structures, mainly the pulmonary veins (PVs) and the posterior left atrial (LA) wall, which are known for trigger activity by means of spontaneous depolarisation and as such initiate AF.
- Fragmentation of the atrial tissue to reduce the critical mass of the conducting tissue to a size that no macro-re-entry circuit, which sustains AF, can exist anymore by the means of self-termination.
- While achieving the above, the Maze principle allows the sinus node impulse to be conducted in a way that atrial and ventricular activation is not affected.
This principle of eliminating the initiation and sustainability of AF in both atria is often called a salvage procedure. However, because the course of AF is a dynamic one with changes over time in conductance velocity (electrical remodeling by a slower or inhomogeneous conductance velocity) and an increase in fibrotic tissue with dilatation of the atrium (anatomical remodeling), a tailored or map-based lesion set might not be necessarily the superior choice, even if available.
It is common and reasonable to treat patients with paroxysmal AF, in which AF initiating triggers reflect the dominant pathology, by isolating the PVs or the PVs and the left posterior wall. However, we must keep in mind that in patients with structural heart disease, one can detect more trigger areas outside the vicinity of the PVs compared to patients with stand-alone AF. In saying this, the milestone study from Haîssaguerre revealing trigger activity being located around the PVs in 90% might not be transferable to patients undergoing cardiac surgery. This is important to understand when evaluating the risk-benefit ratio of a specific lesion set in these patients and applying a certain ablation strategy.
In non-paroxysmal AF, isolation of the PVs will still isolate the triggers but usually fails to interrupt macro-re-entry circuits that sustain the arrhythmia after initiation. In this case, a more extensive lesion set should be applied. Targeting both atria will therefore warrant the highest success because in up to 30% of patients with AF undergoing cardiac surgery, a dominant frequency can be detected in the right atrium. Whether this is mainly related to pathologies causing volume or pressure overload on the right atrium, such as pulmonary hypertension, tricuspid insufficiency, atrial septal defect, etc. is not conclusively proven. Therefore, it is still not possible to clearly identify patients who would benefit from a bi-atrial over an LA only lesion-set and thus could be either over or undertreated. If we lack those identifiers, it is fair to say that a bi-atrial lesion set will warrant best outcome und should therefore be considered in the majority of patients with non-paroxysmal AF. However, in our mitral valve population without any additional pathology affecting the right heart, an extensive LA lesion set only will achieve acceptable success rates because we treat two substrates: the LA macro-re-entrants that sustain AF, and the mitral valve insufficiency or stenosis causing LA remodeling.
- Paroxysmal AF: often trigger-related and therefore focal.
Isolation of areas with a high trigger activity such as the PVs and the LA posterior wall will stop initiation of AF in a high percentage of patients. - Non-paroxysmal AF: macro-re-entry circuit related and therefore non-focal.
A more extensive lesion set is necessary to fragment the atrium in a way that all AF sustaining macro-re-entry circuits are interrupted.
Energy sources
Currently there are two energy sources available that achieve good results in creating transmural lesions: cryo and radiofrequency (RF) energy.
Whilst cryo energy causes cell death by heat retraction, RF energy destroys cells by applying heat. In dose-response studies, both energy sources provide solid performance in creating transmurality over the range of tissue thickness present in the atria. However, both also have their limitations. Being aware of those limitations is critical to warrant best performance by avoiding an environment that would restrict the used device from performing optimally.
Cryo energy
Application of cold temperatures by release of pressurized nitrous oxide or argon gas in cardiac tissues induces formation of ice crystals causing disruption of cell membranes and organelles resulting in inflammation and fibrosis with permanent structural changes. The onset of ice formation at the cryoprobe provides cryoadhesion to maintain and ensure tissue contact and create an area at which heat is extracted from the tissue. As heat is removed, extracellular fluid freezes at −20 ℃, creating a hyperosmotic environment that causes cell shrinkage and, ultimately, cell death. Rapid freezing to −40 ℃ induces expansion of intracellular ice formation, which disrupts organelles and cell membranes even before osmotic imbalance occurs. A fast rate of cooling, which is enhanced by probe material with a good heat conduction coefficient, increases cell death, and slowly thawing the tissue is effective in prolonging the mechanisms of cell destruction, which is exposure-time dependent.
The safety profile is excellent. The quick adhesion of the tissue to the probe allows for retracting the tissue away from adjacent structures. Further, it is the only energy source that preserves collagen and can therefore be applied safely in the vicinity of heart valves.
Application of cryo-energy on the beating heart might limit its performance due to the heat sink effect. The warm intracavity blood distracts fast freezing and counteracts by convection. Moreover, a suboptimal probe-tissue interface with folds might lead to an ice ball formation between the probe and the tissue that decreases freezing performance.
It is therefore crucial to ensure an optimal probe-tissue interface, to have a blood free environment, and to freeze for an adequate time of 120 seconds below a level of −40 ℃ to enhance irreversible cell death and create a transmural lesion along the entire probe length.
RF energy
RF energy uses alternating current in the range of 100 to 1,000 kilohertz (kHz). This frequency is high enough to prevent rapid myocardial depolarization and the induction of ventricular fibrillation, yet low enough to prevent tissue vaporization and perforation. The lesion size created by thermal injury depends on electrode-tissue contact area, the interface temperature, the current and voltage (power), as well as the duration of delivery. On histologic evaluation of RF lesions, a focal coagulation necrosis predominates acutely. This correlates with the irreversible nature of the injury, which occurs at temperatures greater than 50 ℃. There is destruction of the myocardial collagen matrix and replacement with fibrin and collagen in chronic studies. At very high temperatures greater than 100 ℃, char formation predominates.
Due to uncontrolled energy delivery by unipolar RF, this energy source should be applied bipolar. Bipolar RF technology is incorporated into devices in two ways. In one way, the electrodes are embedded in the jaws of a clamp to focus the delivery of energy. By shielding the electrodes from the circulating blood pool, this decreases the heat sink effect, allows for faster ablation times and limits collateral injury to surrounding structures. The second way it is used is unidirectional in a linear device in which the two electrodes are side by side, and the device is applied to either the epicardial or endocardial surface. Bipolar RF ablation clamps are the most reliable devices for creating transmural lesions on the beating heart, both in animals and humans, with short ablation times. Use of the bipolar RF clamp device has eliminated most of the collateral damage that occurred with the unipolar devices, likely due to the focused delivery of energy within the jaws of the clamp, eliminating the diffuse radiation of heat.
Char presents as an impediment to heat transduction and has been associated with asymmetrical ablations. The depth of the lesion can be limited by char formation, epicardial fat, myocardial and endocavity blood flow and tissue thickness. Thus, the electrodes should be cleaned after ablation cycles to remove char that counteracts heat penetration into the tissue.
Direct blood contact of the electrodes might limit its performance and/or disrupt the used algorithm regulating the power according to the measured tissue temperature, conductance or resistance, respectively. Therefore, the device environment should be kept blood free.
- Alternative energy sources have a dose response, and their use should therefore follow certain algorithms or application durations.
- Cryo energy is efficacious in creating transmural lesions after reaching temperatures below −40 ℃ whilst preserving collagen tissue of heart valves. It is therefore the energy of choice for the isthmus lesions that should touch/cross the mitral and/or tricuspid annulus. Depending on tissue thickness, an application duration of minimum 120 seconds per lesion is recommended.
- Bipolar RF clamps are efficacious and safe. They should not be used in the vicinity of heart valves and/or coronaries. The application duration should follow the specified algorithm that is usually based on temperature or tissue conductance measurements and that regulates power application accordingly. Three pairs of ablations (equals six ablation cycles) should best achieve transmurality when using clamps.
- Marking the end of the linear lesions might help to ensure overlap and to avoid gaps.
Predictors of failure
Lack of diligence in using the ablation device in the most effective way and choosing an insufficient lesion set are most probably the two strongest predictors for therapy failure.
However, when reviewing the literature there are two more consistent predictors of failure that should be considered when evaluating the treatment strategy for a given patient: LA size and duration of AF.
Although dilatation of the atria goes along with significant electrical and anatomical remodeling, success rates of surgical ablation decrease disproportionally in LA greater than 6 cm in size. However, this should not be considered as a general cut off. Depending on patient’s characteristics, even larger atria can result in acceptable success rates that are certainly higher than in patients who are left untreated (8).
The same can be said about duration of AF. Although a Maze procedure results in over 90% long term success, this can decrease to around 70% in patients with an AF duration of twenty years. However, the benefit of treatment is still seventy times higher compared to omitting ablation (8).
Thus, increased LA size or long duration of AF reflect no contraindication for surgical ablation. But a thoughtful benefit-risk evaluation becomes more important in patients presenting with these predictors of failure.
- Enlarged LA size and long duration of AF are the most consistent predictors of therapy failure. However, reported success rates in these patients suggest to not use fixed cut-off values but rather apply a more critical benefit-risk evaluation.
Risk assessment
In general, the American Association for Thoracic Surgery consensus statement confirmed that the risk of cardiac procedures is not increased by adding surgical ablation in patients with AF and therefore issues a class I indication for concomitant AF ablation (5). Studies of the Society of Thoracic Surgeons database showed a beneficial perioperative outcome for concomitant ablation even in high-risk patients receiving double or triple valve procedures, a patient group that most surgeons would feel reluctant to extend the procedure by any means (9). Also, patients with low ejection fraction should not be excluded from AF treatment. In fact, this patient group might benefit the most from sinus rhythm restoration and perioperative risk is rather decreased than increased by treating the arrhythmia (10). Moreover, remodeling of the ventricle and improvement of left ventricular ejection fraction occurs significantly more often in AF patients who received surgical ablation.
A complication that is frequently mentioned, especially in the context of the bi-atrial Maze lesion set, is the need for pacemaker implantation. As the lesion set applied does not interfere with the conduction system, in most cases the need for pacemaker is a result of successfully terminating AF and thus unmasking a preexisting sick sinus syndrome, rather than direct damage to the sinus or AV node. However, one should pay attention to apply the cava-caval line laterally to avoid interference with the sinus node complex. This is especially true when using cryo energy, which creates a much wider lesion than RF clamps do. It seems reasonable to wait as long as possible before a pacemaker implantation, because the sinus node that has been damaged by AF recovers in a considerable number of patients over time.
Pulmonary vein stenosis following surgical ablation should be obsolete by making sure a cuff of the atrium is ablated, not the PV themselves.
Phrenic nerve damage is another rare complication of surgical ablation. As the mode of ablation differs in surgical compared to catheter ablation, it is clearly imprudent to freeze the pericardium especially in the vicinity of the pericardial folds when applying the cava-caval line with cryo-energy.
A decision to omit the isthmus lesion connecting the left PV isolation (PVI) to the mitral annulus or an incomplete isthmus lesion might set up the patient for iatrogenic peri-mitral atrial flutter. Although this is still rare, it might be impactful for the patient and is not easy to touch up by endocardial catheter ablation. This is why it is crucial to make sure to touch/cross the mitral annulus with the performed lesion and thus requires cryo energy that does not harm valve tissue. It also requires incorporating the coronary sinus as another possible pathway by either prolonging the endocardial cryoablation to 180 seconds or by ablating the coronary sinus endocardially and epicardially at the same spot.
- Surgical ablation has a favorable benefit-risk ratio even in high-risk patients and/or patients with low left ventricular ejection fraction.
- Perioperative outcome is improved by adding ablation in AF patients.
- The reported complications of surgical ablation, mainly pacemaker implantation, pulmonary vein stenosis and phrenic nerve injury, are usually an unmasked preexisting condition or easily avoidable by taking necessary precautions.
Standard of treatment
Developing a protocol to standardize treatment strategies facilitates reproducibility of results and necessary adjustments, both key factors to improve and to maintain a solid AF program.
The first step is to define indications for surgical ablation of AF. AF in the cardiac surgery population is generally underestimated and underdiagnosed. Heart rhythm assessment is routine in cardiac surgery and should indeed overrule referral letters if applicable. Besides electrocardiogram (ECG), a thorough anamnesis and review of current medication helps to detect undiagnosed or unmentioned AF. Although one single episode of AF under special conditions such as cardiac decompensation did not justify surgical ablation in the past, it is understood that this indicates a certain vulnerability of the atrium and a certain risk for recurrence in the later course even if co-morbidities of the heart are fixed. As such, any documented or confirmed AF in the patient’s history should be considered for treatment.
As stated above, predictors of failures such as LA size and AF duration should be considered when deciding if or how a patient should be treated. Instead of applying specific cut-off values that would comply with the call for standardization, this needs to be evaluated on an individual basis.
Lastly, symptoms are a requirement for interventional AF treatment. However, it is sometimes difficult if not impossible to evaluate if certain symptoms are related to AF or to other cardiac pathologies such as valve or coronary artery disease. Furthermore, midterm complications of AF like stroke, heart failure development or even mortality don’t necessarily have to be correlated to symptoms at the time of surgery. In saying this, symptom free patients with regard of AF should still be candidates for concomitant surgical ablation.
- Surgical ablation is indicated for the vast majority of cardiac surgery patients who present with documented or confirmed AF. Predictors of failure are points of consideration but not general contraindications, neither are conditions increasing the general operative risk such as low left ventricular ejection fraction or complex cardiac procedures.
The second step is to develop an ablation strategy according to the underlying pathology. Without a doubt, the bi-atrial Maze procedure is the most comprehensive lesion set and therefore results in the highest success rates. Still, there may be reasons to deviate from this gold standard. However, this should not be driven by certain access preferences but be based on the underlying mechanisms of AF to not compromise on results that are required for both, applying standard of care as well as sustaining a powerful AF program.
The third step is to make sure patients who are suitable for concomitant AF ablation are identified. This requires awareness of the entire team to pay attention to this arrhythmia and to be knowledgeable of treatment options and their outcomes.
Treatment strategy
The following shows reasonable ablation strategies based on the pathomechanisms and can be applied for any concomitant cardiac surgery with the exception of mitral valve surgery:
- Paroxysmal AF (trigger related—focal)
- PVI using bipolar RF-clamps + LA appendage (LAA) management
- Can be performed epicardially on-pump or off-pump;
- Requires sternotomy.
- Box lesion using bipolar RF-clamps or cryo + LAA management
- Can be performed epicardially on-pump or off-pump (RF) or requires cardioplegic arrest (cryo);
- Requires sternotomy (RF) or a right lateral minithoracotomy (cryo).
- PVI using bipolar RF-clamps + LA appendage (LAA) management
- Non-paroxysmal AF (macro-re-entry related—non-focal)
- Maze procedure (cryo or a combination of cryo and bipolar RF clamps)
- Requires cardioplegic arrest;
- Can be performed via sternotomy or right lateral minithoracotomy.
- Maze procedure (cryo or a combination of cryo and bipolar RF clamps)
In mitral valve surgery either the LA Maze lesion set or a bi-atrial Maze should be performed independent of type of AF, because atriotomy is performed in either case and limitation to PVI or a box only would not significantly reduce time and effort.
LAA management is part of a Maze procedure. It does eliminate a potential trigger source and might therefore also contribute to rhythm control to a minor extent. Even more importantly, it significantly reduces the risk of stroke in AF patients because the main source of thrombus formation is excluded (11).
- If a decision is made to not perform an ablation, at least LAA management should be performed to decrease the AF associated risk of stroke.
The above layout has been a proven treatment strategy that results in good mid- and long-term rhythm outcome and significantly reduces stroke risk and should reflect a solid base to build a sustainable AF program on.
The “access issue”
Reducing invasiveness by avoiding full sternotomy and/or the use of extracorporeal circulation/cardioplegic arrest has been a major goal of cardiac surgery during the last decade. Although the real benefit of those minimally invasive approaches is not entirely conclusive and only proven for certain subgroups, there should be consensus that the chosen technique or access should not limit the application or comprehensiveness of additional therapies that are also indicated and needed. In saying this, the Maze lesion set can hardly be reproduced in an off-pump procedure or partial sternotomy. However, reinforcing those minimally invasive techniques at the cost of the lesion set that would provide best rhythm outcome in a given patient would compromise on providing current standard of care and potentially leaves the patient with an increased risk of stroke, heart failure and mortality.
The implementation of a defined ablation strategy is therefore crucial. The chosen access or technique should then follow this strategy, not vice versa.
The following overview discusses how certain access techniques might limit the applicable lesion set. This of course depends on a surgeon’s expertise and experience and reflects a general assessment suitable for most surgeons:
- Off-pump coronary artery bypass grafting (CABG) via sternotomy
is limited to: - PVI (✓)
- Box-lesion (✓)
- LAA-management (✓)
- Aortic valve surgery via ministernotomy/lateral minithoracotomy is limited to:
- PVI (✓) (challenging)
- Box-lesion (✓) (very challenging)
- LAA-management (✓)
- Mitral valve surgery via right-lateral minithoracotomy:
- Full Maze lesion set including LAA-management (✓)
- All procedures performed via sternotomy using extracorporeal circulation:
- Full Maze lesion-set including LAA-management (✓)
- The chosen access or technique should follow the ablation strategy and lesion set needed to warrant best rhythm outcome. The ablation strategy should not be altered to fit the desired access, because this would compromise standard of care treatment.
Follow-up
A thorough follow-up of patients is mandatory with regard of rhythm outcome after surgical ablation. Although this is sometimes difficult to conduct, only a comprehensive understanding of results allows for adjustments in the applied ablation strategies if necessary. Furthermore, critical decisions such as ceasing oral anticoagulation or antiarrhythmic medication need to be based on rhythm outcome amongst other factors. Moreover, it unmasks the impact of AF to patients that would otherwise remain undetected or at least unknown to the surgeon.
As AF is a dynamic disease, follow-up visits should be scheduled or reliable rhythm information being conducted after a period of three, six, twelve months and annually thereafter.
A twenty-four-hour Holter is the minimum assessment recommended. However, sensitivity is certainly limited by this rather short time frame and should ideally be increased to a seven-day Holter or event recorders.
This should not only allow results to be brought into perspective but also allows comparison to other published reports.
- As true for other cardiac procedures, a thorough follow-up of patients receiving surgical ablation is mandatory to confirm successful results or to adjust the applied protocol as necessary.
Interdisciplinary collaboration
To implement a successful and sustainable AF program, strong communication with the referring cardiologist, electrophysiologist and/or primary care physician is essential. This might even include specialties like neurology when it comes to stroke protection.
They should know of the established treatment protocol and ideally it should be implemented together. This leads to a better understanding of the efficacy and opportunities of surgical ablation. Therefore, it is also important to describe the indications and the pattern of the applied lesion set in the operation report and/or in the discharge letter to facilitate and improve postoperative care or potential re-interventions.
Although a heart team approach is recommended by current guidelines for stand-alone treatment of AF, this should also be applied for concomitant surgical ablation as it is standard for other cardiac procedures.
- Constant communication with other treating specialists such as cardiologist, electrophysiologist and primary care physician regarding established treatment strategies and indications will improve a surgical AF program by all means.
Establishing and expanding a successful AF program
To warrant current standard of care in cardiac surgery it is important to recognize AF as a serious cardiac disease that impacts a significant number of our patient population in terms of stroke, heart failure development and mortality. A protocol to identify patients suitable for surgical ablation and the application of pre-defined ablation strategy will result in good rhythm outcome and ensure any given patient receives accurate treatment. Results need to be checked and should lead to continuous reevaluation of the current treatment protocol. This should be done in close collaboration with other specialties involved in the patient’s treatment.
Once a solid concomitant ablation program is established, this can lead to an expansion that requires even closer collaboration with electrophysiologists such as stand-alone surgical or hybrid ablation, ventricular tachycardia ablation or inappropriate sinus tachycardia ablation (12). This provides opportunities to certain patient subgroups who are challenging to be treated successfully by current endocardial catheter ablation techniques and require a surgeon the electrophysiologist can rely on.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: T.W. declares a financial relationship with AtriCure Inc. The other author has no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCarthy PM, Davidson CJ, Kruse J, et al. Prevalence of atrial fibrillation before cardiac surgery and factors associated with concomitant ablation. J Thorac Cardiovasc Surg 2020;159:2245-2253.e15. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. [Crossref] [PubMed]
- Badhwar V, Rankin JS, Damiano RJ Jr, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg 2017;103:329-41. [Crossref] [PubMed]
- Ad N, Damiano RJ Jr, Badhwar V, et al. Expert consensus guidelines: Examining surgical ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2017;153:1330-1354.e1. [Crossref] [PubMed]
- Iribarne A, DiScipio AW, McCullough JN, et al. Surgical Atrial Fibrillation Ablation Improves Long-Term Survival: A Multicenter Analysis. Ann Thorac Surg 2019;107:135-42. [Crossref] [PubMed]
- Suwalski P, Kowalewski M, Jasiński M, et al. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J Thorac Cardiovasc Surg 2019;157:1007-1018.e4. [Crossref] [PubMed]
- Weimar T, Schena S, Bailey MS, et al. The cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol 2012;5:8-14. [Crossref] [PubMed]
- Damiano RJ Jr, Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 2011;141:113-21. [Crossref] [PubMed]
- Rankin JS, He X, O'Brien SM, et al. The Society of Thoracic Surgeons risk model for operative mortality after multiple valve surgery. Ann Thorac Surg 2013;95:1484-90. [Crossref] [PubMed]
- Khiabani AJ, Schuessler RB, Damiano RJ Jr. Surgical ablation of atrial fibrillation in patients with heart failure. J Thorac Cardiovasc Surg 2021;162:1100-5. [Crossref] [PubMed]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;384:2081-91. [Crossref] [PubMed]
- Doll N, Weimar T, Kosior DA, et al. Efficacy and safety of hybrid epicardial and endocardial ablation versus endocardial ablation in patients with persistent and longstanding persistent atrial fibrillation: a randomised, controlled trial. EClinicalMedicine 2023;61:102052. [Crossref] [PubMed]






