Robotic aortic valve replacement in the Middle East: reproducibility into practice with evolving complexity
Introduction
The management of symptomatic aortic valve disease has continuously evolved, with robotic aortic valve replacement (RAVR) emerging as an innovative, minimally invasive technique (1,2). RAVR offers a sternum- and muscle-sparing approach while still allowing for the use of traditional surgical valves (3). Transcatheter aortic valve replacement (TAVR) has also provided a minimally invasive option for older patients, those at high surgical risk, and individuals with limited life expectancy (4,5). However, the limited durability of TAVR valves, along with challenges related to managing concomitant coronary artery disease, aortopathy, and mitral valve disease, presents significant limitations (6).
Compared with mechanical valves, the use of bioprosthetic valves in younger patients is associated with higher valve-related reoperation rates and reduced long-term survival (7,8). Consequently, practitioners have sought to adopt a minimally invasive approach that also provides patients with durable valves and comparable or superior outcomes to TAVR. Additionally, RAVR has the potential to address concomitant mitral valve disease, reduce the rates of paravalvular leak, and lower pacemaker insertion rates associated with TAVR (6,9). Despite the increasing application of RAVR, longitudinal follow-up data remain limited. This study evaluated the short- and mid-term clinical and echocardiographic outcomes after RAVR.
Methods
Design
We used an ambispective design in which patients who underwent RAVR between March 2022 and November 2024 at King Faisal Specialist Hospital and Research Center were retrospectively recruited and then prospectively followed up. The survival status of all patients was confirmed via telephone contact in November 2024. We included patients who underwent either isolated or concomitant RAVR.
Data collection
All relevant clinical data were retrospectively collected from electronic medical records. The parameters included demographics (age, sex, body mass index), preoperative comorbidities [diabetes mellitus, hypertension, heart failure, pulmonary hypertension, atrial fibrillation (AF), stroke, peripheral arterial disease (PAD), chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD)], symptoms stratified by the New York Heart Association (NYHA) classification, and preoperative echocardiographic data [left ventricular ejection fraction (LVEF), valve pathology, left ventricular end-diastolic diameter (LVEDD), and left ventricular end-diastolic volume (LVEDV)]. Right ventricular dysfunction was defined as tricuspid annular plane systolic excursion (TAPSE) <17 mm (10). Preoperative risk stratification was conducted via the Society of Thoracic Surgery (STS) scoring system. Surgical data included the type of surgery performed (isolated vs. concomitant), the type and manufacturer of the valves, aortic valve size, and aortic cross-clamp time.
Outcome measures
The primary outcomes were in-hospital mortality and survival at follow-up following RAVR. Secondary outcomes included hospital outcomes such as reoperation, blood transfusion, and postoperative complications [acute kidney injury (AKI), new-onset AF, surgical site infection (SSI), and stroke]. Postoperative heart failure was defined as reduced pump function diagnosed based on clinical symptoms, echocardiographic evidence of reduced ejection fraction, and elevated B-type natriuretic peptide (BNP) levels. Hospital complications were defined as those occurring within the same hospital admission or within 30 days of surgery. Readmissions to the intensive care unit (ICU) and hospital were recorded. The LVEF, LVEDD, and right ventricular function were evaluated during follow-up.
Surgical technique
RAVR was performed under general anesthesia using a double-lumen endotracheal tube. Cardiopulmonary bypass was initiated through peripheral cannulation of the right common femoral artery and vein, and the right internal jugular vein. The da Vinci Xi system (Intuitive Surgical, Sunnyvale, CA, USA) was used for the procedure through three ports: a perimammary working and camera port, and left and right ports for robotic arms, placed in the second and sixth intercostal spaces, respectively, along the axillary line.
Venting of the aortic root was achieved through the working port, and a left ventricular vent was placed via the upper right superior pulmonary vein. A transthoracic aortic cross-clamp was applied, and antegrade cardioplegia was delivered via the aortic root. A transverse aortotomy was performed, and the aortic valve was excised via robotic scissors. Circumferentially interrupted 2-0 braided sutures were placed, after which the assistant threaded the sutures through the valve’s sewing ring and delivered the valve through the working port. Suture fasteners (Cor-knot® Device; LSI Solutions, Victor, NY, USA) were used to secure the valve in place, and the aortotomy was closed in two layers.
In cases of RAVR with concomitant mitral valve procedures, the conventional surgical sequence is reversed, prioritizing aortic valve replacement. This adjusted approach deviates from the globally accepted conventional approach of addressing the mitral valve first. The rationale for this shift lies in the surgical access and mitral valve exposure provided by the right mini-thoracotomy robotic approach.
Ethical considerations
The study was approved by the hospital’s Institutional Review Board (IRB #2241086). The study was conducted in accordance with the Declaration of Helsinki (11) and the International Council for Harmonisation (ICH) Harmonized Good Clinical Practice guidelines (12). Owing to the study’s retrospective nature, a waiver of informed consent was granted, and patient anonymity was maintained throughout.
Statistical analysis
Continuous data are presented as means and standard deviations if normally distributed, or as medians and interquartile ranges if skewed. Categorical data are presented as frequencies and percentages. Longitudinal analysis was conducted using a random-effects ordered logistic model for ordinal data, a random-effects model for continuous variables, and the Friedman test for binary data. Statistical analysis was performed using Stata version 18 (StataCorp., College Station, TX, USA), and a P value of <0.05 was considered statistically significant.
Results
Preoperative data
Fifteen consecutive patients underwent RAVR during the study period. The mean age was 38.6±14.4 years, and 86.7% were males. The median STS score was 0.6%. The most common comorbidities were hypertension (46.7%) and diabetes (20.0%). No patients had COPD, PAD, or CKD (Table 1).
Table 1
| Patient characteristics | Data (n=15) |
|---|---|
| Age at time of surgery (years) | 38.6±14.4 |
| Male | 13 (86.7) |
| Body mass index (kg/m2) | 31±7.6 |
| STS score (%) | 0.6 (0.4–1.1) |
| Diabetes mellitus | 3 (20.0) |
| Hypertension | 7 (46.7) |
| Decompensated heart failure | 1 (6.7) |
| Pulmonary hypertension | 2 (13.3) |
| AF | 1 (6.7) |
| Stroke | 1 (6.7) |
| COPD | 0 |
| PAD | 0 |
| CKD | 0 |
| NYHA dyspnea class | |
| II | 7 (46.7) |
| III | 7 (46.7) |
| IV | 1 (6.7) |
| LVEF | |
| ≥55% | 7 (46.7) |
| 50–54% | 7 (46.7) |
| 45–49% | 1 (6.7) |
| Valve pathology | |
| Severe aortic stenosis | 4 (26.7) |
| Severe aortic regurgitation | 7 (46.7) |
| Severe aortic regurgitation + severe mitral stenosis | 1 (6.7) |
| Severe aortic stenosis + severe mitral regurgitation | 3 (20.0) |
| LVEDD (cm) | 5.3±0.9 |
| LVEDV (mL) | 146±50.8 |
Data are presented as mean SD, n (%), or median (Q1–Q3). AF, atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral arterial disease; Q, quartile; RAVR, robotic aortic valve replacement; SD, standard deviation; STS, Society of Thoracic Surgery.
Echocardiographic data revealed that all patients had an LVEF ≥45%, and aortic regurgitation was the most common valve lesion (46.7%). The LVEDD was 5.3±0.9 mm, and the LVEDV was 146±50.8 mL. No patient had preoperative right ventricular dysfunction (Table 1).
Operative data
Isolated RAVR was performed in 66.7% of the patients (n=10), and five patients underwent concomitant surgery, including mitral valve repair (n=1), mitral valve replacement (n=3), and ascending aortoplasty (n=1). Mechanical valves were used in 10 patients (66.7%). The aortic prostheses included On-X (On-X Life Technologies, Austin, TX, USA; n=10), MagnaEase (Edwards Lifesciences, Irvine, CA, USA; n=3), and Perceval (LivaNova, London, UK; n=2). The most common aortic valve size was 23 mm (n=6, 40.0%). No patient required conversion to sternotomy (Table 2).
Table 2
| Type of surgery | Data (n=15) |
|---|---|
| Surgery | |
| Isolated RAVR | 10 (66.7) |
| RAVR + mitral valve repair | 1 (6.7) |
| RAVR + mitral valve replacement | 3 (20.0) |
| AVR + ascending aortoplasty | 1 (6.7) |
| Valve type | |
| Mechanical | 10 (66.7) |
| Biological | 5 (33.3) |
| Valve manufacturer | |
| On-X | 9 (60.0) |
| MagnaEase | 1 (6.7) |
| Perceval | 1 (6.7) |
| MagnaEase + Mosaic | 2 (13.3) |
| On-X + Cosgrove band | 1 (6.7) |
| Perceval + MagnaEase | 1 (6.7) |
| Aortic valve size (mm) | |
| 21 | 2 (13.3) |
| 21/23 | 2 (13.3) |
| 23 | 6 (40.0) |
| 25 | 2 (13.3) |
| 27/29 | 3 (20.0) |
| Ischemic time (min) | 150±33.9 |
| Cardiopulmonary bypass time (min) | 231±55.6 |
Data are presented as n (%) or mean ± SD. AVR, aortic valve replacement; RAVR, robotic aortic valve replacement; SD, standard deviation.
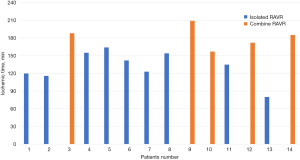
The mean ischemic time was 150±33.9 min, and cardiopulmonary bypass time was 231±55.6 min. Ischemic time decreased markedly after nine cases of isolated RAVR, and it remained unchanged in the fifth combined RAVR compared to the first (Figure 1).
Hospital outcomes
The median length of hospital stay was 9 days [quartile (Q)1–Q3, 4–15 days]. Four patients (26.7%) underwent on-table extubation. The median duration of mechanical ventilation was 72 hours, and the median number of blood units transfused was three. Seven patients (46.7%) underwent reoperation—six for bleeding and one for valve failure.
AKI requiring temporary dialysis occurred in 3 patients (20.0%). AF was reported in 2 patients (13.3%), heart failure in 1 patient (6.7%), and extracorporeal membrane oxygenation (ECMO) was used in 1 patient (6.7%). There were no reported cases of new stroke, permanent pacemaker insertion, SSI, myocardial infarction, or hospital mortality. One patient required ICU readmission because of a low ejection fraction, and one patient was readmitted for pleural effusion (Table 3).
Table 3
| Postoperative outcomes | Data (n=15) |
|---|---|
| On-table extubation | 4 (26.7) |
| Duration of ICU mechanical ventilation (hours) | 72 [6–132] |
| Blood units transfused | 3 [1–8] |
| ECMO | 1 (6.7) |
| ICU readmission | 1 (6.7) |
| Reoperation | 7 (46.7) |
| AKI | 3 (20.0) |
| New-onset AF | 2 (13.3) |
| Heart failure | 1 (6.7) |
| Hospital stay (days) | 9 [4–15] |
| Hospital readmission | 1 (6.7) |
Data are presented as n (%) or median [Q1–Q3]. AF, atrial fibrillation; AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; Q, quartile; RAVR, robotic aortic valve replacement.
Follow-up
The median follow-up was 24 months (Q1–Q3, 15–29 months), and all patients were available for follow-up. No mortality was reported during this period.
All patients had NYHA class I, except for one who had NYHA class II. At the last follow-up, 1 patient (6.7%) had an LVEF <45%, whereas nine patients had an LVEF ≥55%. There were no significant changes in LVEF at the last follow-up compared with the preoperative value (P=0.741). However, the LVEDD was significantly lower at the last follow-up than preoperatively (P=0.003). Two patients had right ventricular dysfunction, which was not significantly different from the preoperative values (P=0.091) (Table 4).
Table 4
| Echocardiography finding | Preoperative | Predischarge | Last follow-up | P |
|---|---|---|---|---|
| LVEF | 0.741 | |||
| ≥55% | 7 (46.7) | 6 (40.0) | 9 (60.0) | |
| 50–54% | 7 (46.7) | 3 (20.0) | 4 (26.7) | |
| 45–49% | 1 (6.7) | 5 (33.3) | 1 (6.7) | |
| <45% | 0 | 1 (6.7) | 1 (6.7) | |
| LVEDD (cm) | 5.3±0.9 | – | 4.7±0.6 | 0.003* |
| Right ventricular dysfunction | 0 | 4 (26.7) | 2 (13.3) | 0.091 |
Data are presented as n (%) or mean ± SD. *, P<0.05. LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; RAVR, robotic aortic valve replacement; SD, standard deviation.
Discussion
Summary of the study
The study evaluated 15 patients who underwent RAVR. Mechanical valves were most commonly used, and five patients underwent concomitant procedures. No hospital or follow-up mortality was reported. Four patients had on-table extubation, and the median duration of mechanical ventilation was 72 hours. There were no reported cases of new-onset stroke, permanent pacemaker insertion, SSI, or myocardial infarction. The median follow-up was 24 months (Q1–Q3, 15–29 months). The LVEDD was significantly lower at the last follow-up compared to the preoperative value.
Comparison with the literature
Minimally invasive cardiac surgery offers several advantages over traditional cardiac surgery, including smaller incisions, faster recovery, less postoperative pain, reduced blood loss, and a lower risk of infection (13-15). RAVR has shown promising outcomes in several recent studies. Badhwar and associates reported outcomes in 20 patients undergoing RAVR (1). The mean age was 68 years, and the most common pathology was aortic regurgitation, similar to our series. They did not report 30-day mortality, renal failure, stroke, reoperation for bleeding, or paravalvular leakage.
The mean age in our patients was lower than in other reports, likely because of the high prevalence of rheumatic heart disease in our region (16). Similarly, Folliguet and colleagues reported their initial experience with RAVR in five patients, noting no sternotomy conversion rate, mortality, stroke, or reoperation for bleeding. The mean ischemic time was 98 min (17).
In a larger series, Wei and colleagues reported outcomes of robotic surgery in 50 patients (2). The median ischemic time was 117 min. Forty-two patients underwent extubation in the operating room, and no operative mortality was reported. Badhwar and associates also reported the initial multicenter experience with RAVR in 212 patients (18). The median age was 67 years, and the median ischemic time was 166 min. Operative mortality was reported in 1% of patients, with reoperation in 8%, renal failure in 1%, and stroke in 1%. The rate of sternotomy conversion was zero.
RAVR has continued to evolve, and a totally endoscopic procedure using sutureless valves has been introduced into the armamentarium of robotic surgery (19). Balkhy and associates reported the first-in-human experience of totally endoscopic RAVR using sutureless valves in a 76-year-old male, who had an uneventful recovery (20).
The learning curve in robotic cardiac surgery is an important consideration when implementing this surgical technique. It has been reported to be longer than that of other surgical procedures. Jonsson and colleagues found that cardiac surgery can be performed safely during the early learning curves; however, mastery may require 250–500 cases, as estimated by the procedure time (21).
Khairallah and collaborators reported that the learning curve, defined by decreased operative time, improved significantly after 30 robotic cardiac cases when expert surgeons moved between centers (22). Wei and associates observed a plateau in ischemic time for RAVR after five cases (2). In our series, ischemic time decreased markedly after nine cases of isolated RAVR; however, it remained stationary after five cases involving concomitant procedures. A longer study is warranted to evaluate the learning curve across more patients.
Several surgical techniques have shown comparable results to their transcatheter alternatives (23). RAVR was introduced as an alternative to TAVR, with the potential to use self-expandable or surgical valves (24). In a propensity score-matched study of 144 patients with low- to intermediate-risk disease, TAVR was compared with RAVR. Conversion to sternotomy was reported in two patients in the TAVR group and in none in the RAVR group (6). Reoperation for bleeding was required in 6% of patients in the RAVR group. However, TAVR was associated with higher rates of heart block, stroke, 1-year mortality, and paravalvular leakage. Therefore, compared with TAVR, RAVR is a minimally invasive approach with a lower complication rate and the possibility of using traditional surgical valves, making it an appealing option for younger patients.
Implications
This study highlights the potential role of the RAVR as a viable, minimally invasive alternative for managing symptomatic aortic valve disease. These findings suggest that RAVR can achieve satisfactory short- and mid-term outcomes, including low mortality rates and acceptable postoperative complication rates. These results could encourage broader adoption of RAVR techniques among surgeons, especially for younger patients who may benefit from the durability of mechanical valves. Furthermore, this study underscores the importance of incorporating RAVR into treatment protocols for patients with concomitant mitral valve disease and other cardiac conditions, where it may offer meaningful advantages over TAVR.
Limitations
This study has several limitations that should be considered. First, the sample size of 15 patients is relatively small, which may limit the generalizability of the findings. Additionally, the ambispective design, which combines retrospective and prospective elements, may introduce biases in data collection and interpretation. Although the median follow-up duration was 24 months, this period may not be sufficient to fully evaluate long-term outcomes and complications associated with RAVR. Finally, the absence of a control group limits the ability to directly compare RAVR outcomes with those of traditional surgical techniques or TAVR.
Conclusions
RAVR demonstrates promising short- and intermediate-term clinical outcomes, positioning it as an effective option for symptomatic aortic valve disease patients. The technique’s minimally invasive nature, along with the ability to use durable mechanical valves, offers potential advantages over traditional surgical approaches. Future studies with larger sample sizes, longer follow-up periods, and control groups are needed to validate these findings further and to assess the long-term outcomes associated with RAVR.
Acknowledgments
None.
Footnote
Funding: None.
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Badhwar V, Wei LM, Cook CC, et al. Robotic aortic valve replacement. J Thorac Cardiovasc Surg 2021;161:1753-9. [Crossref] [PubMed]
- Wei LM, Cook CC, Hayanga JWA, et al. Robotic Aortic Valve Replacement: First 50 Cases. Ann Thorac Surg 2022;114:720-6. [Crossref] [PubMed]
- Sun J, Yuan Y, Song Y, et al. Early results of totally endoscopic robotic aortic valve replacement: analysis of 4 cases. J Cardiothorac Surg 2022;17:155. [Crossref] [PubMed]
- Alabbadi S, Malas J, Chen Q, et al. Guidelines vs Practice: Surgical Versus Transcatheter Aortic Valve Replacement in Adults ≤60 Years. Ann Thorac Surg 2025;119:861-9. [Crossref] [PubMed]
- Thonghong T, De Backer O, Søndergaard L. Comprehensive update on the new indications for transcatheter aortic valve replacement in the latest 2017 European guidelines for the management of valvular heart disease. Open Heart 2018;5:e000753. [Crossref] [PubMed]
- Jagadeesan V, Mehaffey JH, Darehzereshki A, et al. Robotic Aortic Valve Replacement vs Transcatheter Aortic Valve Replacement: A Propensity-Matched Analysis. Ann Thorac Surg 2024;S0003-4975(24)00921-4.
- Alhijab FA, Alfayez LA, Hassan E, et al. Age-Specific Outcomes of Bioprosthetic vs. Mechanical Aortic Valve Replacement: Balancing Reoperation Risk with Anticoagulation Burden. J Cardiovasc Dev Dis 2024;11:227. [Crossref] [PubMed]
- Arafat AA, AlQattan H, Zahra A, et al. Using tissue mitral valves in younger patients: A word of caution. J Card Surg 2022;37:4227-33. [Crossref] [PubMed]
- Darehzereshki A, Wei LM, Comas G, et al. Feasibility and safety of robotic aortic root enlargement in conjunction with robotic aortic valve replacement. JTCVS Tech 2023;22:178-80. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713; quiz 786-8. [Crossref] [PubMed]
- World Medical Association Declaration of Helsinki. ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonized tripartite guideline: Guideline for Good Clinical Practice. J Postgrad Med 2001;47:45-50.
- Akintoye OO, Adu BG, Otorkpa MJ, et al. The current state of minimally invasive cardiac surgery in Africa: a systematic review and meta-analysis. Cardiothorac Surg 2024;32:15.
- Torky MA, Arafat AA, Fawzy HF, et al. J-ministernotomy for aortic valve replacement: a retrospective cohort study. Cardiothorac Surg 2021;29:16.
- Akintoye O, Divya A, Farid S, et al. Sixteen-year outcomes of patients undergoing minimally invasive direct coronary artery bypass surgery: a single-center experience. Cardiothorac Surg 2024;32:16.
- Alsharif ZM, Jawhari AM, Al-halabi SK, et al. Changing trends in Rheumatic heart disease: A retrospective tertiary care hospital-based study in the western region of Saudi Arabia. World Family Medicine/Middle East Journal of Family Medicine 2023;21:6-12.
- Folliguet TA, Vanhuyse F, Konstantinos Z, et al. Early experience with robotic aortic valve replacement. Eur J Cardiothorac Surg 2005;28:172-3. [Crossref] [PubMed]
- Badhwar V, Pereda D, Khaliel FH, et al. Outcomes following initial multicenter experience with robotic aortic valve replacement: Defining a path forward. J Thorac Cardiovasc Surg 2024;167:1244-50. [Crossref] [PubMed]
- Nagaoka E, Gelinas J, Vola M, et al. Early Clinical Experiences of Robotic Assisted Aortic Valve Replacement for Aortic Valve Stenosis with Sutureless Aortic Valve. Innovations (Phila) 2020;15:88-92. [Crossref] [PubMed]
- Balkhy HH, Kitahara H. First Human Totally Endoscopic Robotic-Assisted Sutureless Aortic Valve Replacement. Ann Thorac Surg 2020;109:e9-e11. [Crossref] [PubMed]
- Jonsson A, Binongo J, Patel P, et al. Mastering the Learning Curve for Robotic-Assisted Coronary Artery Bypass Surgery. Ann Thorac Surg 2023;115:1118-25. [Crossref] [PubMed]
- Khairallah SM, Rahouma M, Mick SL. Transferring Surgical Expertise: Analyzing the Learning Curve of Robotic Cardiac Surgery Operative Time Reduction When Surgeon Moves from One Experienced Center to Another. J Cardiovasc Dev Dis 2024;11:81. [Crossref] [PubMed]
- Arafat AA, Zahra AI, Alhossan A, et al. Comparison of Outcomes After Transcatheter Versus Surgical Repeat Mitral Valve Replacement. Braz J Cardiovasc Surg 2023;38:52-61. [Crossref] [PubMed]
- Suri RM, Burkhart HM, Schaff HV. Robot-assisted aortic valve replacement using a novel sutureless bovine pericardial prosthesis: proof of concept as an alternative to percutaneous implantation. Innovations (Phila) 2010;5:419-23. [Crossref] [PubMed]






