Advancing robotic aortic valve replacement beyond isolated therapy: a platform for multivalve therapy
Introduction
The right transaxillary 3 cm working incision used for robotic aortic valve replacement (RAVR) is nearly identical to the platform used for robotic mitral operations. The familiarity of this platform has helped to facilitate the addition of concomitant procedures following initial cases of RAVR as an isolated operative procedure starting in January 2020 (1). While some concomitant procedures were novel and required careful planning, the majority were readily adaptable to maintain procedural consistency with commonly applied robotic operations. Following RAVR initiation, concomitant procedures were incorporated within the first 50 cases at West Virginia University, to include mitral valve (MV) repair and biatrial Cox-Maze operations (2). Upwards of 17% of the first 300 global cases of RAVR have safely included concomitant operations with excellent outcomes up to one year (3).
Operative techniques
Preparation
Following preoperative assessment of coronary and vascular anatomy with contrast computed tomography (CT), and angiography when deemed necessary, transthoracic, or transesophageal assessment of valvular function is performed on all patients. Following induction with double lumen endotracheal anesthesia, central line and left brachial arterial line placement, an 18–20 French cannula is percutaneously inserted via the right internal jugular vein under echocardiographic guidance and positioned in the superior vena cava (SVC) to facilitate future bicaval drainage. All patients are positioned supine with a shoulder roll under the tip of the right scapula and the right arm is tucked posteriorly with the humerus as vertical as possible to expose the right axilla (Figure 1). Following skin prep and draping, a 3 cm transaxillary working incision is performed at the level of the anterior axillary line in the fourth intercostal space and a soft tissue retractor is placed. The pericardium is entered 3–4 cm anterior to the phrenic nerve and brought laterally by way of retraction sutures brought out at the level of the posterior axillary line to create a shelf-like exposure of the cardiac structures. The DaVinci Xi robot (Intuitive Surgical, Sunnyvale, CA, USA) is prepared. Port placement preparation is made in relation to the working incision as follows: arm 1 at 10 o’clock in the third intercostal space, arm 3 at 1 o’clock in the fifth intercostal space, arm 4 at 4 o’clock in the sixth intercostal space, with arm 2 placed via the working incision when the time comes.
A 1 cm skin incision is placed slightly above the right inguinal crease to access the anterior surface of the right common femoral artery and vein; 5-0 polypropylene purse strings are placed, and systemic heparin is given. A 25 French multi-stage venous cannula is delivered into the right atrium, and a 17–19 French arterial cannula is placed. Bicaval cardiopulmonary bypass is commenced. An aortic root vent is placed on the distal lateral aorta (at the level of the SVC) and brought through the working incision. A left ventricular vent is placed via the right superior pulmonary vein and brought out through a separate stab incision posterior to the working incision. A transthoracic cross-clamp is introduced through a stab incision in the second or third intercostal space at the anterior or mid axillary line and positioned distal to the aortic root vent. The robot is docked with the port configuration noted above with instruments introduced as follows: arm 1—DeBakey, arm 2—30 degree up camera, arm 3—long tip forcep grasper, arm 4—curved scissors (Figure 2).
Exposition
The table-side surgeon or associate applies the cross-clamp and cardioplegia is delivered directly into the aortic root to achieve hypothermic diastolic arrest in the setting of less than moderate aortic regurgitation (AR). Should AR be moderate or greater, it is recommended to proceed with aortotomy and deliver cardioplegia via direct coronary ostial catheters. The aortotomy, when performed, is most commonly done in a modified “hockey stick” fashion (Figure 3), commencing approximately 2 cm distal to the sinotubular junction and extending medially and cephalad and laterally and caudad to the mid non-coronary sinus to facilitate excellent exposure of the aortic valve.
In the setting of aortic stenosis without significant AR and the need to perform concomitant intracardiac operations, it would be advised to commence with the left atriotomy first. This would be the case of a RAVR and concomitant MV repair or replacement, and/or concomitant Cox-Maze with cryoablation. A vertical right atriotomy provides access for the right-sided Cox-Maze cryoablation lesions as well as to provide exposure for concomitant tricuspid valve (TV) repair or replacement. In the setting of significant AR, it is advised to open the aorta and deliver ostial cardioplegia before embarking upon intracardiac operations. We have not used retrograde cardioplegia in these settings due to several factors that include logistical considerations, but also anatomic as in the case of the Cox-Maze III lesion set and importance of performing the epicardial coronary sinus lesion.
Operation
All operations should be performed in alignment with open or previously established robotic procedures to ensure standardization and patient safety. In the case of concomitant left atrial operations performed first, the initial instrument in arm 3 is the dual blade atrial retractor and is retained until such time that aortotomy is necessary. Once the left atrium is opened, the left ventricular vent is positioned into the left inferior pulmonary vein. If a concomitant mitral repair or replacement is the only additional procedure, this should proceed as per institutional routine, with placement of the left ventricular vent across the completed MV prior to left atrial closure facilitated by 4-0 polytetrafluoroethylene. If a concomitant biatrial Cox-Maze procedure is the only additional procedure, the left-sided lesion set should proceed first, followed by the right-sided lesion set, followed by left atrial appendage ligation (4). If mitral or tricuspid procedures are needed in addition to the Cox-Maze, the Cox-Maze is performed first in the order noted above, followed by either the MV and then the TV to allow for adequate tissue thawing. Following closure of the left and right atriotomies, the RAVR is then performed. These steps can facilitate double valve and even triple valve operations, with or without concomitant surgical ablation in an efficient and safe manner (5).
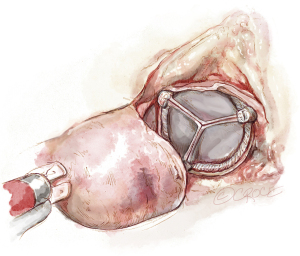
Following completion of the aortotomy, aortic valvectomy is performed with the robotic curved scissors commencing at the right-non commissure and then extending either along the right or non-coronary annulus per the surgeon preference (Figure 4). It should be noted that in all of our cases, the robotic curved scissors provide the precision and dexterity needed to perform complete and accurate debridement, flush to the annulus and without the need for a rongeur. Tableside suction aspiration and meticulous irrigation is essential to ensure complete evacuation of any and all particulate matter (Figure 5). Circumferential interrupted braided sutures are placed from the ventricular side starting at the left-non commissure and moving clockwise. Left-handed placement, often just using the DeBakey, may assist with seamless delivery of sutures along the non-coronary annulus by avoiding any inadvertent interaction with the non-coronary sinus. The annulus is then sized, and the valve sutures are then systematically placed through the prepared prosthesis extracorporeally and then the prosthesis is delivered through the working incision, often off the holder to fit through the incision, and then lowered into position carefully with robotic assistance. The valve is then secured with suture fasteners to complete the replacement (Figure 6), and the aorta is closed robotically with 4-0 polypropylene suture in two layers or per institutional routine (Figure 7A). Antegrade cold blood or cardioplegia is then administered to not only provide additional myocardial protection if required, but to test the suture line prior to removal of the cross-clamp (Figure 7B).
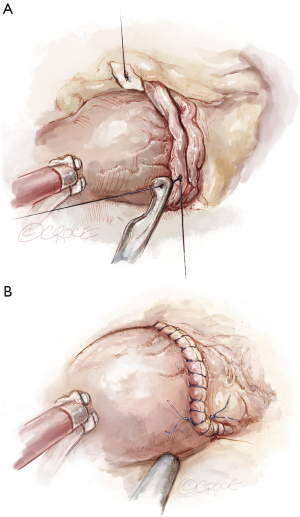
A guiding principle of Should be denoted at (SAVR) as per standard nomenclature, and thus of RAVR as well, is to always implant the largest size prosthesis possible, matched to the patient’s body surface area to avoid mismatch. Shortly after the launch of RAVR, we performed our first aortic root enlargement on May 12, 2020 (6). Since that time, root enlargement has been performed in 10% of all cases worldwide (3). The technique utilized commences by extending the aortotomy to the annulus at the midpoint of the non-coronary sinus in a modified Nick’s fashion, with optional extension along a portion or the entirety of the annulus of the non-, then completing the aortic prosthetic implantation (Figure 8). While not an annular enlargement per se, enlargement of the aortic root often affords upsizing by 1–2 sizes. Reconstruction is then performed with either autologous or bovine pericardium, or synthetic polyester, often determined by the size of the augmentation required. Our preference is to utilize synthetic polyester for larger enlargements. The patch is fashioned to size and lowered through the working incision where it undergoes additional refinement of size (Figure 9). The first suture is placed outside-in at the base of the aortic tissue, at the level of the annulus, and then up through the patch (Figure 10). Each limb of the 4-0 polypropylene is then sutured rightward, then leftward, and tied together to complete the augmentation, often of the entire aortotomy to provide maximum advantage (Figure 11). The integrity of the augmentation is then tested with antegrade cold blood or cardioplegic delivery.
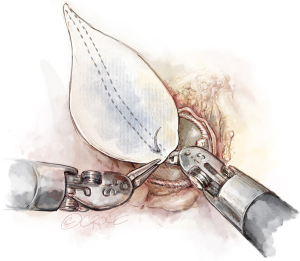
Additional options for concomitant procedures include extended transaortic septal myectomy for the management of hypertrophic cardiomyopathy. Following valvectomy, the visualization and access to the entirety of the left ventricular outflow tract is both clear and coaxial. This enables a complete myectomy extending well towards the ventricular apex and beyond the mitral leaflet and papillary muscle continuity in order to provide a thorough resection using only the robotic curved scissors.
Finally, a single-incision, single-platform via the right transaxillary approach may be possible to perform concomitant RAVR and coronary artery bypass grafting. The left internal thoracic artery can be harvested via the right approach. Following myocardial arrest, the left pericardium is marsupialized to facilitate cardiac rotation and exposure of the left anterior descending to permit totally endoscopic coronary artery bypass (7).
Completion
Upon completion of the RAVR and concomitant intracardiac operations, epicardial temporary pacer wires are placed, and the heart is re-animated in conjunction with cross-clamp removal. The pericardium is closed, and chest drains are placed. The patient is fully ventilated on both lungs while on full cardiopulmonary bypass, then weaned from support and closed. Operating room extubation is then performed per institutional protocols or exchanged to single lumen intubation and transferred to the intensive care unit. Postoperative management is continued per institutional routine.
Comments
Robotic cardiac surgeries and the institutions performing them will continue to evolve through the expansion of their armamentarium, the safe addition of cases of increased complexity, and the addition of concomitant procedures. The establishment of RAVR in January 2020 was done intentionally using the same transaxillary working incision as robotic mitral operations (1). The aim was to be able to add concomitant operations as experience grew. This added flexibility permits RAVR to be applied to an increased cohort of patients who may benefit from more than isolated aortic valve surgery. The addition of concomitant procedures to RAVR became feasible within the first 6 months, with the first biatrial Cox-Maze on May 1, 2020 (4). To avoid patient-prosthesis mismatch, the first concomitant root enlargement with patch augmentation was performed on May 12, 2020 (6). The first concomitant RAVR with MV repair and biatrial Cox-Maze was performed on July 24, 2020 (8). MV replacement for aortic and mitral stenosis was performed on March 16, 2021, and the first transaortic septal myectomy for hypertrophic cardiomyopathy was performed on December 8, 2022 (9). Many of these concomitant procedures, or combinations thereof, have become routine with growing RAVR team experience with multicenter reproducibility (3,9).
Operative experience and team building is essential for the safe introduction of these added procedures. Robotic cardiac surgery, starting predominantly with MV repair, has been well-established in our program, with over 1,000 cases performed by our team before developing RAVR. Excellence in preoperative imaging, anesthesia, perfusion, and seamless coordination between console and tableside surgery has been instrumental to permit the relatively standardized additions of concomitant procedures. The same platform is used for all robotic valve cases and the vast majority of our patients are extubated in the operating room, thus enhancing our ability to perform these operations timely, often with two operations by the same team in a day, and in a cost-neutral manner to conventional sternotomy (10). After overcoming the learning curve for robotic mitral surgery, additional concomitant procedures such as AVR, root enlargement, complex bileaflet mitral repair, endocarditis repair, biatrial Cox-Maze, and TV repair are all possible with the same platform. As long as a patient is suitable for peripheral cardiopulmonary bypass, a relative all-comer strategy is employed at our institution. Every patient is evaluated by the heart team, which now considers RAVR as the preferred approach for low-risk and even intermediate-risk patients with isolated aortic valve disease as well as when concomitant procedures are required.
Conclusions
In conclusion, as experience grows with RAVR, the addition of concomitant procedures and multi-valve operations is both feasible and safe. Utilizing the same 3 cm right transaxillary approach, robotic surgery is no longer limited to a single-procedure modality.
Acknowledgments
None.
Footnote
Funding: None.
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Badhwar V, Wei LM, Cook CC, et al. Robotic aortic valve replacement. J Thorac Cardiovasc Surg 2021;161:1753-9. [Crossref] [PubMed]
- Wei LM, Cook CC, Hayanga JWA, et al. Robotic Aortic Valve Replacement: First 50 Cases. Ann Thorac Surg 2022;114:720-6. [Crossref] [PubMed]
- Wei LM, Pereda D, Ramzy D, et al. Longitudinal outcomes following international multicentre experience with robotic aortic valve replacement. Eur J Cardiothorac Surg 2025;67:ezaf103. [Crossref] [PubMed]
- Almousa A, Mehaffey JH, Wei LM, et al. Robotic-assisted cryothermic Cox maze for persistent atrial fibrillation: Longitudinal follow-up. J Thorac Cardiovasc Surg 2023;165:1828-1836.e1. [Crossref] [PubMed]
- Comas GM, Wei LM, Badhwar V. Robotic-assisted double valve surgery. Ann Cardiothorac Surg 2022;11:543-4. [Crossref] [PubMed]
- Darehzereshki A, Wei LM, Comas G, et al. Feasibility and safety of robotic aortic root enlargement in conjunction with robotic aortic valve replacement. JTCVS Tech 2023;22:178-80. [Crossref] [PubMed]
- Badhwar V, Raikar GV, Darehzereshki A, et al. Robotic-Assisted Aortic Valve Replacement and Coronary Artery Bypass Grafting. Ann Thorac Surg 2025;119:918-22. [Crossref] [PubMed]
- Badhwar V, Wei L. Robotic aortic valve replacement with concomitant mitral valve repair and bi-atrial Cox maze. CTSNet. February 24, 2022. doi:
10.2573/ctsnet.19232997 . Available online: https://www.ctsnet.org/article/robotic-aortic-valve-replacement-concomitant-mitral-valve-repair-and-bi-atrial-cox-maze - Badhwar V, Pereda D, Khaliel FH, et al. Outcomes following initial multicenter experience with robotic aortic valve replacement: Defining a path forward. J Thorac Cardiovasc Surg 2024;167:1244-50. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]














