Total endograft replacement of aortic arch
Introduction
Surgical treatment of aneurysmal disease affecting the aortic arch is complex and is associated with significant risk of morbidity and mortality. Operative strategies can be summarized as a traditional open approach, conventional hybrid endovascular approach and most recently a near total endograft option. Open repair mandates the use of hypothermic circulatory arrest and a sternotomy, which is a durable option that allows the treatment of the ascending aorta as well as arch pathology. Any necessary cardiac procedures, such as valve replacement or coronary bypass surgery, can be performed at the same time. In cases associated with distal arch and descending thoracic aortic pathology, the approach is limited by accessibility through a median sternotomy. In these circumstances, arch reconstruction with distal elephant trunk is usually undertaken with a planned second stage procedure. Often, this second procedure is performed as an open operation via a left lateral thoracotomy. Alternatively, a hybrid endovascular approach may be employed by placing a thoracic endograft into the ‘elephant trunk graft’.
The conventional hybrid approach is to surgically ‘de-branch’ the aortic arch with a bifurcated graft taken from the ascending aorta and anastomosed distally to the proximal supra-aortic vessels, usually in conjunction with a left carotid subclavian bypass. This then permits endovascular exclusion of the arch aneurysm with a conventional thoracic endovascular stent graft with its proximal seal zone just distal to the takeoff of the bifurcated graft (1).
Total endovascular repair of the aortic arch is a complex endovascular procedure with promising early results (2). The ability to branch into major aortic branches has evolved following experience in the iliac system and the para-visceral aorta. Clear advantages include the avoidance of a sternotomy, avoidance of cumbersome extra-anatomic debranching, and preclusion of the need for hypothermic circulatory arrest.
Operative techniques
The aortic arch presents complex spatial geometry with curves and three-dimensional angulations that may be extremely challenging. This produces problems related to the apposition and long term integrity of endovascular devices. Inadequate apposition of the stent graft to the inner curve of the aortic arch may cause postoperative endoleaks.
Following placement, stent grafts are subject to great dynamic strain as a result of the curved configuration, high blood flow, and pulsatile movement of the aorta.
The standard considerations in any stent graft procedure apply to the arch branch procedure.
These include the following:
- Suitable access, to allow a 24 Fr outer diameter system to be placed in the aortic arch. Iliac arteries should be greater than 8 mm in diameter and there should be an acceptable degree of tortuosity in the aortic arch, descending thoracic aorta, abdominal aorta, and iliac arteries;
- Appropriate proximal and distal sealing zones of greater than 20 mm length are required. The distal sealing zone will vary greatly on a case by case basis but the proximal diameter in the ascending aorta must be equal to or be less than 38 mm, in order to effect a stable seal;
- The branches are routinely placed in the innominate artery, which should have a minimum diameter of 8 mm, and the left common carotid artery, which should have a minimum diameter of 6 mm.
Three device-specific concepts make this procedure technically possible.
- Curved Nitinol delivery cannula;
- Notched delivery system;
- Internal side branches.
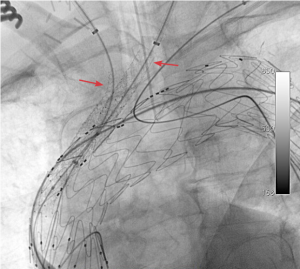
The pre-curved nitinol delivery cannula has the unique ability to reliably orient itself within the arch (Figure 1). Aided by relatively predictable position of the arch vessels, this makes the procedure possible without the need for rotational movement of the device once in the arch. Indeed, rotational adjustment is virtually impossible once the device reaches its intended site of deployment. The orientation notch in the dilator tip is aligned with the outer curve of the graft (Figure 2). In addition to the radio-opaque branch markers, this allows for more accurate confirmation of orientation and positioning. Internal side branches are accessed through diamond shaped orifices and extend for 2 cm parallel to the aortic graft (Figure 3).

Case presentation
79-year old male present with a 6.5 cm aortic arch aneurysm and an associated 5.4 cm descending and thoracoabdominal aneurysm.
Previous infra-renal abdominal aortic aneurysm was repaired with a bifurcated graft. This original procedure was complicated by occlusion of the left limb of the bifurcated graft, requiring a right-to-left femoral crossover graft which was itself complicated by a large seroma. In order to avoid the seroma and potentially infecting the new endograft, common iliac access via a unilateral retroperitoneal exposure was planned.
The standard pre-operative workup for a complex endograft was performed. In addition, the carotid arteries were included in the CT scan to exclude significant supra-aortic disease. Echocardiography was performed, in particular to assess for aortic valve pathology.
The nose cone of the deployment cannula by design crosses the aortic valve. Significant valvular disease on pre-operative imaging is therefore a relative contraindication.
Finally, a close working relationship is necessary between the entire extended team, including anaesthesia, interventional cardiology, nursing staff and X-ray technicians.
Access and adjunctive bypass procedures
A left carotid to subclavian bypass is routinely required with the current generation of graft. In this particular case, due to a short innominate artery, sealing was planned bilaterally in the common carotid arteries. This necessitated bilateral carotid-subclavian bypass. Due to the presence of seromas access for the endograft was via direct common iliac puncture. After exposure of the common iliac artery, 2 concentric purse string sutures were placed using 5-0 Prolene. Bilateral carotid-subclavian bypasses were performed (Video 1). The platysma was divided, the clavicular head of the sternocleidomastoid muscle was transected, and the subclavian artery exposed with control of branches as required. The common carotid artery was isolated and a tunnel created behind the internal jugular vein. Following systemic heparinisation and clamping, a 7 mm Dacron graft was anastomosed in an end-to-side fashion to the subclavian artery using 5-0 Prolene. Following restoration of flow to the arm, the graft was placed behind the internal jugular vein and the common carotid artery clamped in preparation. An arteriotomy in the common carotid artery was performed followed by end-to-side anastomosis again using 5-0 Prolene. Wire access was gained through the common iliac artery purse string. A long 7 Fr sheath was advanced into the descending thoracic aorta. The aortic valve was then crossed using interventional cardiology techniques. This is usually achieved with a pigtail catheter or a Glide wire. Once across the aortic valve, a double curve long Lunderquist wire was then placed deep within the left ventricle.
Comment: the bridging stents are usually placed via access in the proximal common carotid artery on the left and via either the axillary or common carotid artery on the right. These are usually inserted following surgical exposure. Our preference is to place 6 or 7 Fr ‘Brite Tip’ sheaths through a purse string. The sheath tip is placed just distal to the aortic arch to mark the vessel and provide a target for graft positioning. It should be noted that the innominate bridging stent is usually a custom Cook limb. The carotid branches can be done using any covered stent, either self expanding such as Viabahn (Gore) or balloon expandable such as Advanta V12 (Atrium). However, in this case, because we were branching into each of the common carotid arteries, we chose to use the new flexible Advanta V12 (Atrium) stent grafts.
Endograft positioning
The delivery system was advanced over the Lunderquist wire into position. Inspection of the orientation notch and branch markers was used to confirmed satisfactory orientation. Digital subtraction angiography was performed through a pigtail catheter inserted via one of the carotid sheaths. The branch markers were aligned with the supra-aortic branches with the aid of the Brite Tip sheath markers at the ostia of the supra-aortic vessels.
Comment: before advancing the delivery system, it is our practice to check the effectiveness of our rapid pacing wire at producing a satisfactory level of hypotension. At this point of the operation, the nose cone will have crossed the aortic valve. The graft markings indicating the openings of the internal branches must be examined to ensure that the external opening of the branch is accessible from the supra-aortic vessels. Ideally, the branch should be positioned just proximal to the target supra-aortic vessel.
Endograft deployment
Following a final angiogram to check position and mark the screen, rapid ventricular pacing was commenced and the graft deployed under hypotensive conditions with the systolic blood pressure below 60 mmHg.
Comment: the graft is deployed under asystolic conditions. Our preference is for rapid ventricular pacing as it offers rapid onset and resolution. The standard Cook system, whereby the sheath is retracted to expose the graft, is used. We first withdraw the sheath to the proximal margin of the fabric before commencing rapid pacing and final deployment. Often, the friction during this initial withdrawal is the most difficult and requires noticeable strength. The procedure can be paused once reaching this level in order to prepare for the final rapid subsequent deployment.
The final deployment is performed with firm (slightly forward) pressure on the deployment cannula to prevent the graft from being displaced distally by a windsock effect. If this is allowed to happen the branches may not be accessible, therefore highlighting the importance of asystole during rapid pacing. Sequential release of the device is achieved by pulling the first three control rings on the deployment handle. The first belongs to the spiral stabilising wire. The second ring removes the inner curve attachments of the proximal stent. The third ring releases the proximal diameter reducing ties and outer curve attachment of the proximal stent. Rapid pacing can be discontinued after release of the third ring. Note that there is a fourth release ring that removes the distal diameter reducing ties and distal attachment to the delivery system. This is usually released after all necessary extensions have been placed. The nose cone can be withdrawn from the left ventricle at this stage by loosening the pin-vice and retracting the tip until it reaches the pusher.
One should always be aware of the risk of covering either a native coronary artery or the origin of a coronary bypass graft during placement. If this happens, cardiac arrest or severe cardiac ischemia will occur and the graft must be pulled distally.
Place bridging stents
The branches were cannulated sequentially. To confirm position within the branch, a pigtail catheter was inserted over the wire and the formed pigtail was withdrawn. Deformation of the pigtail catheter at the branch confirmed position. This was repeated for the second branch. The carotid sheathes were placed into the branches, followed by the bridging stents (Advanta V12 by Antrium). The left carotid branch was reinforced with a self-expanding stent because of tortuosity (Figure 4).
Comment: the usual pattern is to place covered stents into the left carotid with either a balloon-expandable or self-expanding, covered stent. The innominate artery distally is usually too large for a standard-size covered stent and requires a customized graft. This is usually a modified iliac limb placed on a shorter delivery system. It can be introduced via the axillary or carotid artery depending on which route is more favorable. In this case, the short sealing zone in the innominate artery required bridging into the right common carotid artery.
Completion
The final release wire on the control handle was pulled, detaching the branch graft completely from the delivery system. The device was anchored by inflating angioplasty balloons in the bridging stents at the level of the internal branches while the nose cone and delivery system was withdrawn. The endograft was then extended into the distal sealing zone using TX2 grafts. An Amplatzer plug was deployed via the left brachial artery in the proximal left subclavian artery.
Comment: withdrawal of the nose cone and delivery cannula carries significant risk of displacing the branch graft. We therefore fixed the carotid branches in position by inflating angioplasty balloons within the Atrium stents at the site of overlap with the internal branches, maintaining inflation as the device was withdrawn. Similarly, advancing additional extension thoracic stent grafts may displace the device proximally. In this case, we simply performed this step cautiously as we had unilateral iliac access only. If bilateral access is possible, the extension piece can be advanced into the branch graft prior to release of the final trigger wire. This prevents proximal displacement of the arch branch graft while introducing the distal extension.
The proximal left subclavian artery requires occlusion to prevent a type 2 endoleak. In this case the right side was also occluded by ligation during the carotid subclavian bypass operation.
Comment on complications
Potential complications include access complications, injury to the left ventricle and aortic valve, renal failure, endoleak, embolisation, retrograde dissection, and death. Additionally, stroke, cardiac ischaemia, and spinal cord ischaemia are critical complications.
Stroke is the major Achilles heel of this procedure. The three main mechanisms that cause particular concern are embolisation, thrombosis and low cerebral flow during implantation.
Additionally, immediate cardiac ischaemia can occur due to coverage of the origin of a coronary bypass graft. This mandates pulling the graft distally. If this prevents access to the innominate branch, an extra-anatomical bypass is then required.
Finally, spinal cord ischaemia is a significant concern. We try whenever possible to separate final aortic coverage in very extensive repairs to ameliorate this risk. Despite this, we have experienced one case of paraplegia which we suspect was a result of extensive embolic showering.
Discussion
The list of potential operative complications from this procedure mandates an extremely cautious approach. In our experience embolic stroke has been the most troublesome complication. There is a significant amount of instrumentation in the arch and supra-aortic vessels. We have experienced both minor and major strokes and are now experimenting with our sheath positioning to allow placement of embolic protection devices during the procedure. Additionally, we are wary of significant aortic thrombus and supra-aortic occlusive disease.
The large delivery system presents some fairly predictable issues in relation to access, as well as lower limb ischaemia concerns. We have experienced rhabdomyolysis in one case and now practice a temporary shunt technique.
While initial results worldwide are relatively sparse, they show significant promise. Low risk patients with no history of cardiac procedures should be offered conventional open or hybrid surgery. The arch branch procedure should only be considered in patients deemed high risk for conventional treatment or in patients for whom difficult redo surgery is anticipated.
Acknowledgements
Disclosure: Simon Neequaye, none; Cherrie Z. Abraham, paid consultant to Cook Medical for case proctoring and review.
References
- Andersen ND, Williams JB, Hanna JM, et al. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg 2013;57:655-67; discussion 666-7. [PubMed]
- Lioupis C, MacKenzie KS, Obrand DI, et al. Treatment of aortic arch aneurysms with a modular transfemoral multibranched stent graft: initial experience. Eur J Vasc Endovasc Surg 2012;2:525-32. [PubMed]