Why is the mammary artery so special and what protects it from atherosclerosis?
Introduction
The internal mammary arteries (IMAs) are commonly used as the conduit to bypass major coronary artery stenosis, and have shown greater long-term patency rates and improved survival as compared to saphenous vein grafts (SVGs) (1,2). The benefit of IMAs over SVGs on mortality has been consistently observed irrespective of age, gender, degree of luminal stenosis in the left main coronary artery or preoperative left ventricular function with the survival differences widening over time (3). The main differences are related to the development of atherosclerosis which has rarely been observed in the IMA graft while it develops at a fairly rapid rate in the SVG (4,5)
Since the publication by Loop et al. in 1986 on 10-year survival of patients who received an IMA graft to the left anterior descending coronary artery (LAD) with or without one or more vein grafts versus patients who received only SVGs, which showed that the survival was higher with an IMA graft (93.4%) versus SVG (88.0%) for those with one-vessel disease, 90.0% versus 79.5% for two-vessel disease, and 82.6% versus 71.0% (P<0.0001) for those with three-vessel disease, the IMA has become the preferred choice for grafting the LAD (6). SVGs are known to undergo not only intimal thickening but also atherosclerosis, and angiographic studies demonstrated a 2% per year vein-graft attrition rate from the 1st to the 7th postoperative year, further increasing to 5% per year from the 7th to the 12th year (5). At 10-years, it has been reported that only 38% to 45% of SVG remain patent (6,7). These studies have helped document the superiority of IMA graft over SVG.
Comparative anatomy of IMA and SVG
The IMA is an elastic artery which arises from the subclavian artery. In adults the diameter of the IMA varies from 1.9 to 2.6 mm, with a wall thickness of 180 to 430 microns (8). The intima consists of endothelium with some neointima, which is seen in up to 50% of cases and rarely (13%) is there a substantial neointima which is greater than the medial thickness (8). The media consists of discreet lamellae of collagen and smooth muscle cells (SMCs) that are located between the elastic layers and are aligned circumferentially. The number of elastic layers varies from 7 to 11, depending upon the thickness of the wall of the IMA. The adventitia has been shown to possess very few vasa vasorum (9,10). On the other hand, the SVG has a larger diameter (3.1 to 8.5 mm) and its wall thickness ranges from 180 to 650 microns. The vein has longitudinally oriented bundles of SMCs in the inner media and adventitia and the circumferentially oriented medial cells are in between the longitudinal fibers. Type I collagen separates the longitudinally oriented SMC bundles and is also interspersed between the circumferentially oriented SMCs. Elastic lamellae are observed in the intima, media and adventitia; in the latter, the fibers are interspersed between collagen fibers. In the intima, there is no prominent internal elastic lamina; however, multilayered appearance is observed with interspersed SMC and collagen. Intimal thickening has been described to be almost always seen in vein grafts at the time of implantation; however, in 90% of cases it occupies <25% of the cross sectional area (8,11).
Histologic changes observed in long-term IMA graft versus SVG
It is well known that SVGs are susceptible to accelerated atherosclerosis as compared to native coronary arteries or IMAs, thus limiting the long term benefits of coronary artery bypass graft (CABG) surgery with SVGs. SVGs at the time of implantation show focal absence of endothelium with platelet and fibrin deposition along the intimal surface. Acute inflammatory cells are often observed in the wall of the graft. Vein grafts in place for more than one month show diffuse intimal thickening consisting of SMCs, proteoglycans and collagen. The mechanisms responsible for rapid neointimal growth in SVGs are believed to involve response to endothelial injury along with hemodynamic stress as the vein wall is now subjected to arterial pressures. SVGs implanted for over one year show arterialization and fibrointimal thickening that consists of SMCs, proteoglycans, and type I and III collagen. The degree of intimal thickening varies and is reported to be usually <75% cross-sectional area narrowing with <10% demonstrating >75% narrowing from neointimal tissue only (11). Patients with fibrointimal proliferation have been shown to have higher systolic and diastolic blood pressure (12). Atherosclerotic change in vein grafts has been observed as early as 13 months. The earliest change consists of foam cell accumulation overlying neointimal thickening, which is observed close to the luminal surface and is usually extensive. Foam cell accumulation is soon followed by the presence of a necrotic core, which is observed between 1 to 3 years. SVGs implanted for more than five years frequently exhibit a large necrotic core, with hemorrhage that likely occurs both from the lumen and less often from adventitial neoangiogenesis extending into the intima, leading to expansion of the necrotic core and eventually plaque rupture (4).
The changes of atherosclerosis in SVGs also correlate with the presence of risk factors as they do for native coronary arteries. We have shown a good correlation of total cholesterol with the development of vein graft atherosclerosis. Aggressive treatment with lovastatin achieving LDL-cholesterol (LDL-C) <100 mg/dL resulted in only 27% of grafts with progression of atherosclerosis while moderate treatment resulted in 39% of grafts with progression (13), and low dose warfarin therapy had no effect on atherosclerosis. Although much progress has been made in the understanding of SVG disease to increase survival, the best results, even with aggressive lipid lowering do not parallel those with the use of IMA grafts (14).
Early IMA graft failure is most commonly attributed to technical errors with harvesting and the graft anastomosis. IMA grafts examined within the first week following distal anastomosis show an absence of neointimal thickening or there are only a few SMC along with proteoglycan and collagen. When IMA grafts are examined between 1 week and 2 months, the site of the anastomosis shows intimal thickening (0.08±0.07 mm) located at the cleft between the native artery and the IMA graft at the anastomotic suture site (Figure 1) (15). The intimal thickening consisted of SMCs, proteoglycan, collagen and elastin fibers with luminal endothelial cells. However, in the body of the graft at this time, there are only occasional areas that show minimal intimal thickening consisting of a few SMCs in a proteoglycan matrix with or without collagen, likely due to manipulation of the artery at the time of surgery. Significant intimal thickening was observed in grafts implanted for 2 months to 10 years at the suture sites (0.39±0.17 mm) and on the hood (0.29±0.25 mm), while intimal thickening on the floor (native LAD) was observed in 10 of 18 IMA grafts (0.11±0.12 mm) (Figure 2). Intimal thickening is similar in those grafts less than 1 year versus grafts greater than 1 year, suggesting that intimal thickness does not increase with time. The body of the IMA graft also showed the least intimal thickening as compared to the anastomotic site (10 of 18, 0.03±0.04 mm). Only rarely was an atherosclerotic change observed in the IMA. In our study, 2 of the 18 grafts examined 5.22±4.76 years following grafting, it was described as “small focal, infiltrates of lipid in the intima”.
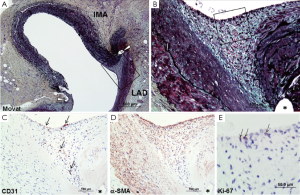
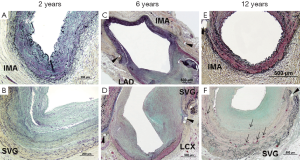
Our published long-term morphologic data in IMA grafts versus SVGs was reported in 1988 (5), where 18 IMAs were compared to 15 SVGs from 18 patients with duration of grafts between 12 to 118 months (mean, 56 months) that had been removed either surgically or at necropsy. We found that fibrointimal proliferation alone was more frequent in IMAs as compared to SVGs [IMA; 8 of 18 (44%) versus SVG; 4 or 15 (27%)]. However, since vein grafts beyond one year are often accompanied by foam cell infiltration, with or without a necrotic core, such changes were observed in 9 of 15 SVGs (60%). In contrast, atherosclerosis was extremely rare in IMA grafts and was only observed in 1 of 18 IMA (6%) (vs. SVG, P=0.01) and that too consisted of only a few foam cells within the neointimal tissue in a graft which showed severe stenosis at the anastomotic site at 3 years.
Not only has the left IMA graft to the LAD been demonstrated to remain patent and improve longevity at 10- and 15-years, bilateral IMA has an additional effect of reducing myocardial infarction, reoperation and percutaneous coronary interventions (PCI) (16). Similarly, skeletonization of the IMA had no effect on long-term patency, but added extra length. However, this also carries the possibility of decreased risk of deep sternal infection, which is likely related to significant postoperative reduction in sternal perfusion (17). Bilateral IMA are also increasingly being used as Y- or T-composite arterial grafts for treating 3-vessel coronary artery disease. The short-term blood flow reserve results have been good (18), with long-term data required to confirm patency.
What protects IMA from atherosclerosis?
The superiority of IMAs over SVGs with less mortality and greater patency rates (>90% at 10 years) could be attributed to the striking resistance of this conduit to atheroma, where multiple structural and physical properties of the IMA could be involved (Table 1) (19). It is interesting to note that IMA grafting of the LAD is also associated with less progression of native atherosclerotic disease within the proximal LAD as compared to when a vein graft is anastomosed to the LAD, as well as greater and rapid native disease progression from the development of fibrosis and calcification.
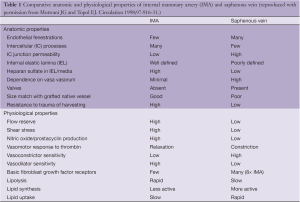
Full table
Role of endothelial cells in IMA patency
The IMA endothelium shows fewer fenestrations and lower intercellular junction permeability as compared to SVG, which could prevent lipoproteins from entering the subendothelial space. Segments of IMA collected at the time of surgery show a preserved morphology without any disturbance of endothelial cells or cautery burns, with uniform platelet endothelial cell adhesion molecule-1 (PECAM-1) staining, and strong expression of glucose transporter 1. Conversely, inducible nitric oxide synthase (iNOS) and intercellular adhesion molecule-1 (ICAM-1) are only moderately expressed on the luminal surface as well as on vasa vasorum of IMAs removed from patients with acute coronary syndrome or chronic stable angina (20). Endothelial cells of the IMA are rich in heparin sulfate and endothelial nitric oxide synthase (eNOS), and release a greater amount of nitric oxide (NO) that contributes to the antithrombotic properties and endothelial homeostasis which confers protection from atherosclerosis.
It has been reported that female sex is a well-defined independent predictor of poor outcome following bypass grafting. The differences between men and women have been attributed to technical and anatomic factors, especially smaller body size with smaller coronary artery size. It has been shown that age-related impairment of NO production is likely enhanced in post-menopausal women and is ascribed to the loss of the protective effects of estrogens. Recently, Mannacio et al. (21) have shown that IMA endothelial cells from menopausal women have impaired expression of messenger RNA for eNOS and reduced eNOS protein levels as compared to age matched men and younger women.
Blood flow and the endothelium
Blood flow creates two principal vectors on the vessel wall, one which is perpendicular to the wall and is determined by the blood pressure, and the other which is parallel to the vessel wall and creates frictional force and shear stress on the endothelial cells. Endothelial cells align in the direction of flow but the orientation is lost with flow disturbances. The stress on the surface of the endothelial cells leads to cytoskeleton changes that attach the endothelial cell to the subendothelial matrix and to adjacent cells, leading to increased resistance to deformation and impart stability. Endothelial cells sense shear-stress and are the principal endothelial regulator of arterial diameter, which may be related to the release of NO. Other substances that also mediate vaso-regulation include prostaglandin I2, endothelin-1, tissue plasminogen activator, ICAM-1, and transforming growth factor-β1 (TGF-β1). While the effects of NO are short-lived, NO synthesis is enhanced by the steady laminar flow that induced eNOS. The arterial remodeling seen in the IMA occurs over months and is a response to flow that result in changes in gene expression. Chronic flow increase results in enlargement of the arterial lumen whereas reduced flow induces intimal thickening and a reduction in vessel lumen. This has been demonstrated in the canine IMA following reduction of flow by ligation of the side branch (22). The IMA has an abundant collateral blood supply to its runoff bed, which also lead to the protection of the intima (23). Furthermore, the size of the IMA is close to the size of the coronary vessels, which may result in less turbulent flow as compared to the larger SVG conduits that are prone to develop atherosclerosis.
SVG must undergo adaptive changes (“arterialization”) when placed in the high pressure aorta-coronary circulation, whereas the IMA is already accustomed to the high left-sided circulation pressures. Flow reserve in the IMA is higher than that in SVGs, and the IMA graft may have the ability to enlarge substantially over years (24). Porto et al. (25) have recently reported the results of quantitative coronary angiography (QCA) and frequency-domain optical coherence tomography (FD-OCT) to determine long-term morphofunctional remodeling of left IMA (LIMA) grafts versus in situ right IMA (RIMA) in the same patients with the duration of the LIMA grafts more than 10 years. Baseline mean diameter and area of LIMA grafts were significantly smaller than that of in situ RIMA. FD-OCT revealed that the LIMA as compared to the RIMA had a larger mean intimal area [LIMA =0.50 mm2 (0.37-1.02 mm2) versus RIMA =0.30 mm2 (0.20-0.45 mm2); P=0.05] and greater maximal intimal thickness [LIMA =156.5 µm (77.7-186.2 µm) versus RIMA =45.0 µm (29.0-70.3 µm); P=0.01] with a non-significant medial thinning. Intimal-media ratio was greater in LIMA as compared to RIMA [LIMA = 0.72 (0.53-0.91) versus RIMA =0.23 (0.12-0.38); P=0.02]. Furthermore, endothelium-dependent and independent vasodilation was tested by selective infusion of acetylcholine (ACh) and isosorbide dinitrate (ISDN), where vasodilatory response as determined by percentage increase of mean lumen diameter did not differ between the LIMA and the RIMA. Despite the intimal thickening present in the LIMA, both the LIMA and the RIMA have a similar response to vasodilators. Although a mismatch between vasodilatory response and intimal thickening was only observed in LIMA, this might represent an adaptive response to different flow pattern encountered in LIMA versus RIMA following CABG.
Coronary artery bypass grafting (CABG) as compared to percutaneous coronary intervention (PCI)
CABG has been the treatment of choice compared to balloon angioplasty since results from the Bypass Angioplasty Revascularization Investigation (BARI) trial in 1996, of patients with multi-vessel coronary disease, showed patients with CABG lived longer than patient undergoing balloon angioplasty (26). Since the advent of drug-eluting stents (DES), interventional cardiologists have contended that CABG may not be superior to stenting because of improved results with DES over bare metal stents or balloon angioplasty. With the use of 1st-generation DES in the SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study, it was shown that all cause death or myocardial infarction was not different in the two arms; however, repeat vascularization was significantly more frequent in PCI than CABG (13.5% vs. 5.9%, P<0.001) in the first year. The differences in myocardial infarction and repeat PCI have now been both shown to be significantly lower for CABG as compared to PCI at 3- and 5-years. These results are also similar to those in the recently published ASCERT study (27), which was a large non-randomized observational data from The Society of Thoracic Surgeons and the American College of Cardiology Foundation registries to evaluate effectiveness of revascularization with CABG compared to PCI. This too showed a benefit for CABG with a 4-year mortality of 16.4% in the CABG arm versus 20.8% in the PCI arm (risk ratio, 0.79; 95% confidence interval 0.76-0.82) (27). Similar results have also been published for the FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial. The primary outcome of composite of death from any cause, non-fatal myocardial infarction, or nonfatal stroke occurred more frequently in the PCI than in the CABG group (P=0.005), with 5-year rates of 26.6% in the PCI and 18.7% in the CABG group (28). Both the SYNTAX and the FREEDOM trials had over 94% of patients undergoing left IMA to LAD grafting, thus showing that even with much improvement in the DES, patients with multi-vessel disease should undergo CABG preferably with as many arterial grafts as possible (however this was not demonstrated in the trials). Although many patients do not want a sternotomy, it is possible to advocate a minimally invasive direct coronary artery bypass surgery (MIDCAB), which has shown promising results in expert surgical hands (29). It is also possible to carry out hybrid procedures with IMA to LAD and DES in right or the circumflex coronary arteries, as indicated. This will require better cooperation between the surgeons as well as the interventionist, although this has not been true in the past. However, with the advent of transcatheter aortic valve replacement (TAVR), there appears to be greater cooperation and more of an atmosphere of congeniality. If we take the oath as physician “The health of my patient will be my first consideration” to heart then everyone will win; the surgeon, the interventionalist, the cardiologist, and the patient (30).
Conclusions
There is little doubt that the IMA is a superior graft than the saphenous vein, so it behooves all thoracic surgeons to take the time and care to carry out CABG with an IMA and to use as many arterial grafts as possible. We may not have all of the information as to why the IMA graft is far superior to vein grafts, but this understanding is increasing. A better comprehension of the uniqueness of the IMA’s mechanical factors, such as shear stress forces, endothelial cell attachment with tight endothelial junctions and biochemical generation of important molecules like nitric oxide and anti-thrombotic factors, and resistance to the generation of selectins and other adhesion molecules, may help to understand why the IMA is resistant to the development of atherosclerosis. Similarly, we need to understand why the SMC of the IMA are resistant to proliferation and remain phenotypically contractile for decades. With increased understanding, it is more likely that we will be able to transfer such knowledge to the prevention of atherosclerosis in native arteries too.
Acknowledgements
CVPath Institute Inc., Gaithersburg, Maryland, USA provided full support for this work. Dr. Otsuka is supported by a research fellowship from the Uehara Memorial Foundation, Tokyo, Japan.
Disclosure: Dr. Virmani receives research support from Abbott Vascular, BioSensors International, Biotronik, Boston Scientific, Medtronic, MicroPort Medical, OrbusNeich Medical, SINO Medical Technology, and Terumo Corporation; has speaking engagements with Merck; receives honoraria from Abbott Vascular, Boston Scientific, Lutonix, Medtronic, and Terumo Corporation; and is a consultant for 480 Biomedical, Abbott Vascular, Medtronic, and W.L. Gore. Dr. Sakakura has received speaking honorarium from Abbott Vascular, Boston Scientific, and Medtronic CardioVascular. The other authors report no conflicts.
References
- Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts--effects on survival over a 15-year period. N Engl J Med 1996;334:216-9. [PubMed]
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a department of veterans affairs cooperative study. J Am Coll Cardiol 2004;44:2149-56. [PubMed]
- Loop FD. Internal-thoracic-artery grafts. Biologically better coronary arteries. N Engl J Med 1996;334:263-5. [PubMed]
- Yazdani SK, Farb A, Nakano M, et al. Pathology of drug-eluting versus bare-metal stents in saphenous vein bypass graft lesions. JACC Cardiovasc Interv 2012;5:666-74.14.
- Shelton ME, Forman MB, Virmani R, et al. A comparison of morphologic and angiographic findings in long-term internal mammary artery and saphenous vein bypass grafts. J Am Coll Cardiol 1988;11:297-307. [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [PubMed]
- Campeau L, Enjalbert M, Lespérance J, et al. The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. A study 10 years after aortocoronary bypass surgery. N Engl J Med 1984;311:1329-32. [PubMed]
- Canham PB, Finlay HM, Boughner DR. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovasc Res 1997;34:557-67. [PubMed]
- Galili O, Herrmann J, Woodrum J, et al. Adventitial vasa vasorum heterogeneity among different vascular beds. J Vasc Surg 2004;40:529-35. [PubMed]
- Galili O, Sattler KJ, Herrmann J, et al. Experimental hypercholesterolemia differentially affects adventitial vasa vasorum and vessel structure of the left internal thoracic and coronary arteries. J Thorac Cardiovasc Surg 2005;129:767-72. [PubMed]
- Waller BF, Roberts WC. Remnant saphenous veins after aortocoronary bypass grafting: analysis of 3,394 centimeters of unused vein from 402 patients. Am J Cardiol 1985;55:65-71. [PubMed]
- Atkinson JB, Forman MB, Vaughn WK, et al. Morphologic changes in long-term saphenous vein bypass grafts. Chest 1985;88:341-8. [PubMed]
- The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. The post coronary artery bypass graft trial investigators. N Engl J Med 1997;336:153-62. [PubMed]
- Kulik A, Ruel M. Lipid-lowering therapy and coronary artery bypass graft surgery: what are the benefits? Curr Opin Cardiol 2011;26:508-17. [PubMed]
- Ojha M, Leask RL, Johnston KW, et al. Histology and morphology of 59 internal thoracic artery grafts and their distal anastomoses. Ann Thorac Surg 2000;70:1338-44. [PubMed]
- Burfeind WR Jr, Glower DD, Wechsler AS, et al. Single versus multiple internal mammary artery grafting for coronary artery bypass: 15-year follow-up of a clinical practice trial. Circulation 2004;110:II27-35. [PubMed]
- Damgaard S, Steinbrüchel DA, Kjaergard HK. An update on internal mammary artery grafting for coronary artery disease. Curr Opin Cardiol 2005;20:521-4. [PubMed]
- Affleck DG, Barner HB, Bailey MS, et al. Flow dynamics of the internal thoracic and radial artery T-graft. Ann Thorac Surg 2004;78:1290-4; discussion 1290-4. [PubMed]
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 1998;97:916-31. [PubMed]
- Foglieni C, Maisano F, Dreas L, et al. Mild inflammatory activation of mammary arteries in patients with acute coronary syndromes. Am J Physiol Heart Circ Physiol 2008;294:H2831-7. [PubMed]
- Mannacio V, Di Tommaso L, Antignano A, et al. Endothelial nitric oxide synthase expression in postmenopausal women: a sex-specific risk factor in coronary surgery. Ann Thorac Surg 2012;94:1934-9. [PubMed]
- Barner HB. Remodeling of arterial conduits in coronary grafting. Ann Thorac Surg 2002;73:1341-5. [PubMed]
- Kay HR, Korns ME, Flemma RJ, et al. Atherosclerosis of the internal mammary artery. Ann Thorac Surg 1976;21:504-7. [PubMed]
- Bashour TT, Hanna ES, Mason DT. Myocardial revascularization with internal mammary artery bypass: an emerging treatment of choice. Am Heart J 1986;111:143-51. [PubMed]
- Porto I, Gaudino M, De Maria GL, et al. Long-term morphofunctional remodeling of internal thoracic artery grafts: a frequency-domain optical coherence tomography study. Circ Cardiovasc Interv 2013;6:269-76. [PubMed]
- Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The bypass angioplasty revascularization investigation (BARI) investigators. N Engl J Med 1996;335:217-25. [PubMed]
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012;366:1467-76. [PubMed]
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375-84. [PubMed]
- Holzhey DM, Cornely JP, Rastan AJ, et al. Review of a 13-year single-center experience with minimally invasive direct coronary artery bypass as the primary surgical treatment of coronary artery disease. Heart Surg Forum 2012;15:E61-8. [PubMed]
- WMA declaration of Geneva. Available online: http://www.wma.net/en/30publications/10policies/g1/





