Harvesting the radial artery
Introduction Other Section
The radial artery (RA) has emerged as an important arterial graft for coronary bypass surgery. Five-year patency rates in more recent studies are better than 85% (1). Key parameters influencing the long-term patency of the RA graft are appropriate patient selection, meticulous coronary target selection and scrupulous operative technique. In this article we review the steps in harvesting the RA via either an open or endoscopic approach. Prerequisites of a successful harvest include adherence to important anatomical landmarks, protection of the sensory innervation to the volar forearm, and meticulous handling of the RA branches (2). This discussion will focus on the features of the forearm anatomy that are most relevant to the surgeon. For a more complete discussion of forearm anatomy, the reader is referred to the more expansive discussion published previously by one of the authors (2). Table 1 lists the abbreviations used in this article.
Full table
Operative techniques Other Section
For the surgeon harvesting the RA, the pertinent anatomy can be summarized by the following phrase: “two muscles, two nerves, and two branches (Figures 1,2)”. See Table 2 for a listing of these anatomic structures. The two muscles are the brachioradialis muscle (BRM) and the flexor carpi radialis muscle (FCRM). These muscles, along with their interconnecting fascia, describe the crevice wherein lies the RA. The two nerves are the lateral antebrachial cutaneous nerve (LABCN) and the superficial radial nerve (SRN). These are the nerves most prone to injury during RA harvesting and knowledge of their course will minimize the risk of injury. The two branches, the recurrent radial artery (RRA) and the superficial palmar artery (SPA), define the proximal and distal limits of the RA harvest respectively. Further details regarding these anatomic features will be discussed below.


Full table
There are two basic approaches for harvesting the RA: the open approach and the endoscopic approach. Each of these will be described in turn. Please see Table 3 for a summary outline of the alternative operative approaches, dissection planes, and branch-handling techniques.

Full table
For all operative approaches, the arm is prepped circumferentially, draped, and secured to an arm board that is positioned at no more than 90 degrees with respect to the operative table. If both a mammary artery and a RA are being harvested, it is useful to simultaneously harvest each mammary artery along with the contralateral RA. If only one RA is harvested, usually the non-dominant arm is chosen.
A preoperative modified Allen’s test is conducted. In this test, the patient makes a clenched fist, and the radial and ulnar arteries are compressed firmly at the wrist by the examiner. While compression is maintained, the patient slowly opens the wrist and incompletely extends the fingers (hyperextension can produce a false positive result). When the ulnar artery is released, a hyperemic response extending to the thenar eminence and thumb within 5 seconds indicates adequate collateral circulation by the ulnar artery and non-dominance of the RA (3).
Other useful adjuncts for preoperative RA evaluation include duplex examination and pulse oximetry. In general, because of concern over vasospasm, we avoid RAs measuring less than 2 mm in diameter.
Open approach for radial artery harvest
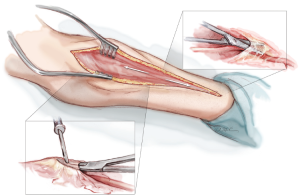
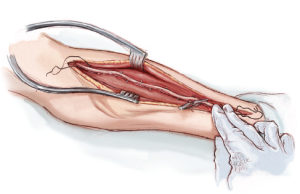
A curvilinear skin incision, tailored to the edge of the brachioradialis muscle, extends from 1 cm distal to the elbow crease to 1 cm proximal to the wrist crease (Figure 1). Corroboration of the appropriate position of the skin incision can be obtained by palpating the radial pulse proximally and distally. Proximally, the radial pulse is best appreciated within the inverted V formed by the biceps tendon laterally and the bicipital aponeurosis medially. This inverted V also defines the site where the radial recurrent artery (RRA) branches off from the RA. Distally, the RA can be palpated between the radial styloid laterally and the tendon of the flexor carpi radialis medially.
Once through the skin, superficial veins are either retracted or divided between clips. Next, the fascia overlying the RA is incised as the RA emerges to become a subcutaneous structure from beneath the belly of the BRM in the mid-forearm (Figure 2). This will expose the RA and its venae comitantes lying in loose areolar tissue. The fascia is divided more proximally with electrocautery, separating the muscle bellies of the BRM and the FCRM. Distally, the fascia is divided with sharp scissors due to the close proximity of the underlying RA here.
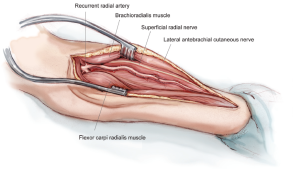
There are two nerves that are of consequence during the RA harvest: the LABCN and the SRN (Figure 3). These nerves provide cutaneous innervation to the volar forearm, portions of the thumb and the dorsum of the hand (2). The LABCN, a branch of the musculocutaneous nerve, lies within the superficial fascia overlying the BRM, and will retract from the field of view once the intervening fascia between the BRM and the FCRM is divided. It frequently travels in proximity to the cephalic vein (4). The SRN travels lateral and in close proximity to the RA. With the appropriate amount of tissue retraction—just enough to visualize the course of the RA—both of these nerves are well protected and less likely to be injured. In fact, the nerves are often not seen at all, a desirable state of affairs.
Once the plane of the RA pedicle is entered, the dissection is carried proximally and then distally. We prefer harvesting the vessel as a pedicle, along with its venae comitantes. However, others recommend either skeletonization (5) or extrafascial harvesting (6). See Table 3. We feel that the pedicle technique minimizes manipulation of the RA, decreases operative time, and facilitates RA dissection. Regardless of the dissection technique chosen, the RA should be handled with great care at all times, if it is handled at all.
A useful maneuver once the RA is exposed is to soak a sponge in papaverine solution (3 mg papaverine per mL of saline) and lay it over the portion of the RA that is not being addressed at any point in time. For example, during dissection of the proximal RA, the sponge should lie over the distal RA, and vice versa. Periodically, additional papaverine solution is added to the sponge so as to adequately bathe the RA.
Key internal landmarks for the proximal and distal limits of RA harvesting are two of its major branches (Figure 3). Proximally, the RA should be harvested to just below the takeoff of the RRA. This will not only preserve the collateral network communicating with the RRA, but will also keep the surgeon in safe territory. Important structures vulnerable to injury reside proximal to the RRA, including the ulnar artery, brachial artery and median nerve. Distally, the artery should be harvested proximal to the takeoff of the superficial palmar artery (SPA). This preserves the radial-ulnar collateral network to the hand. While the RRA can be seen within the confines of the incision, the SPA is usually hidden from view distally. Generally speaking, if a shorter segment of RA is needed, the more proximal vessel segment is chosen due to its less developed muscularis layer. This will minimize the effect of vasospasm.
The RA gives off numerous intervening perforating branches that supply the forearm and hand. Most of the branches arise from the dorsal hemicircumference of the RA; in fact, branches are almost never seen arising anteriorly. Proximally under the belly of the BR muscle, an average of just over 4 branches is found. Distally, where the RA is a subcutaneous structure, more than twice as many branches are encountered and are most numerous near the wrist. Besides being more abundant, the more distal branches are shorter and more delicate than the more proximal branches, making them more challenging to dissect out and transect (7).
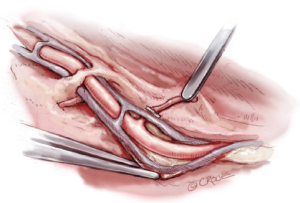
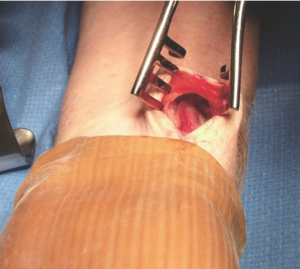
A variety of alternative techniques are available to manage these RA branches. These techniques are listed in Table 3 and include (I) electrocautery alone; (II) sharp dissection with clips; (III) a combination of electrocautery and clips and (IV) ultrasonic dissection. We prefer to use a combination of electrocautery and clips for the rapidity with which the vessel can be harvested and reliability of controlling larger branches. With this technique, a clip is used to secure the branch abutting one of the veins, and electrocautery is used to divide the vessel distally towards the tissue (Figure 4). Despite concerns about heat generation from the electrocautery, injury can be minimized by keeping the electrocautery current low (20 Watts). A recent study from the Texas Heart Institute revealed that, properly harvested, the RA does not sustain any intimal injury with the electrocautery at a low setting, and graft flows are actually higher in comparison to those harvested with the sharp dissection technique (8). Regardless, the jury is still out as to which technique, if any, results in better preservation of the RA architecture and hence greater long-term patency of the graft.
Importantly, the RA should never be grasped directly; its venae comitantes provide a convenient and safe grasping surface when handled prudently (Figure 4). Another practical method for retracting the RA is to gently roll it with a papaverine-soaked gauze to one side while managing its branches. Critically, the RA should never be stretched to improve exposure, as stretching causes separation of the intima from the vessel wall.
Once the vessel’s branches have been transected and the RA has been mobilized, the RA is atraumatically clamped in its distal portion to confirm a retrograde pulsation from the ulnar collateralization (Figure 5). After adequate collateral flow is confirmed, the vessel is ligated with a heavy silk tie distally and transected. The proximal vessel is similarly ligated and transected. The proximal RA is carefully cannulated with a 2 mm flexible olive-tip cannula, and the vessel is flushed with vasodilating solution under minimal pressure. The contents of this solution at our institution are listed in Table 4. The vessel is then soaked in the same solution until later use. Subsequently, when cardioplegia is initiated, the RA is connected to the cardioplegia apparatus and cardioplegia is delivered down the graft to detect any side branches that require additional clips.

Full table
Once the RA is removed from the arm, the arm is closed in its most superficial layers only. The deeper fascial layers are left unapproximated to minimize the risk of compartment syndrome and nerve injury. The subcutaneous layers are closed as per convention.
Endoscopic technique for radial artery harvest
Endoscopic Radial Artery Harvest (ERAH) has been rising in popularity in recent years as a result of increasing familiarity with endoscopic vein harvesting and expanding use of the RA as a conduit. The specific ERAH technology chosen depends on the experience of the harvester and the individual institution. Prepping and positioning are the same as for the open technique.
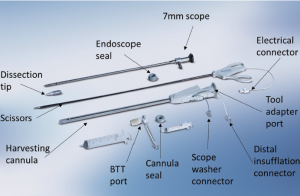
There are two categories of systems for ERAH: the open system and the sealed system. The open system uses a specialized retractor for endoscopic exposure, but CO2 is not delivered in a pressurized fashion, as the system remains open to the atmosphere. The closed system delivers CO2 insufflation at a controlled pressure to aid visualization; the wound is sealed at the scope entry site with a specialized balloon. The authors are familiar with the latter technique using the Vasoview Endoscopic Vessel Harvesting System (Maquet) and this is the approach that will be described below (Figures 6,7).
A 3 cm longitudinal incision is made over the RA, ending 1 cm proximal to the wrist flexion crease. The RA and its venae comitantes are identified under direct vision (Figure 8). The fascia overlying the pedicle is divided with scissors as far proximally as possible under direct vision to create room for scope entry.
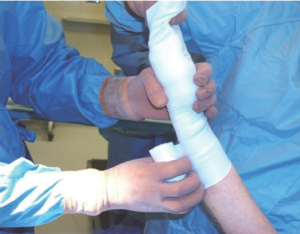
A sterile tourniquet is then applied to the upper arm, and the entire arm is wrapped tightly with a sterile Esmark bandage from its distal to proximal end (Figure 9). The sterile tourniquet is inflated to 75 mmHg above the systolic pressure (not to exceed 200 mmHg), and the Esmark removed. This will create a bloodless field. It is important to complete the open distal RA exposure prior to tourniquet application to minimize ischemic time. The start and stop times of tourniquet inflation should be noted and recorded, and every effort expended to keep its duration under 60 minutes.
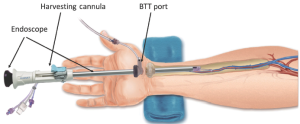
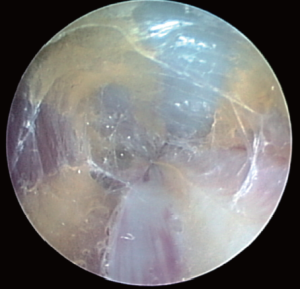
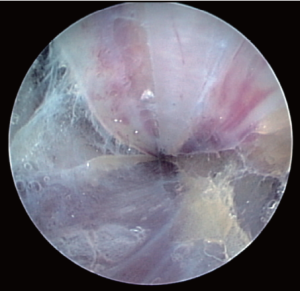
The components of the Vasoview System can be seen in Figures 6,7. Figure 6 depicts the complete component set for one of the more recent generations of the Vasoview System. Figure 7 shows the Harvest cannula inserted into the blunt-tipped trocar (BTT) port in situ in a human arm. To commence the dissection with the Vasoview System, the clear bullet tipped dissector is threaded on to the scope tip. The blunt-tipped trocar (BTT) is then pre-loaded onto the scope, and the dissector is advanced anteriorly over the RA. Once the dissector is advanced approximately 3 cm, the BTT is slid down over the scope into the incision. The BTT contains a balloon that is inflated with sequential 5 cc aliquots of air (up to 25 cc) until a seal is created (Figure 7). The gas line is connected to the insufflation port, and CO2 is insufflated at a rate of 3-5 L/min under a pressure of 10-12 mmHg. The dissector is then used to bluntly dissect the RA and its venae comitantes as a pedicle from the surrounding tissue. The dissector is advanced anteriorly (Figure 10), withdrawn, then advanced posteriorly (Figure 11), and withdrawn once again. Significantly, whenever the dissector is advanced, actual contact with the RA itself should be avoided if possible; accordingly, the dissector is biased slightly to either side of the RA during advancement, so that any contact made is with the venae comitantes instead. In addition, the scope should be slightly torqued away from the pedicle, transmitting any forces to the surrounding tissue. Finally, as the dissection proceeds and branches are encountered, tissue should be judiciously cleared around the branches and the branches themselves should be minimally displaced. The dissector advancement should be up to the level of the RRA or the venous plexus in the antecubital fossa, depending on the scope’s relative position with respect to the RA.
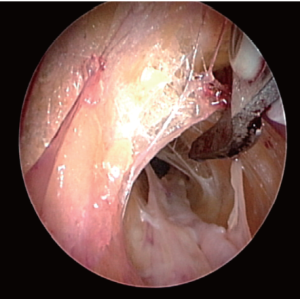
Once the dissection is complete, the scope is withdrawn, the dissection tip is removed from the scope and the scope is then inserted into the harvest cannula. The harvest cannula contains several ports through which different tools can be inserted and advanced (Figures 6,7). Via the harvest cannula, the cautery instrument can be introduced to perform a fasciotomy of the BRM-FCRM fascia (Figure 12). This will create space to facilitate the harvest and to reduce the risk of compartment syndrome developing in the forearm postoperatively. A cautery instrument is then used to divide the side branches of the RA, while a vessel cradle keeps the RA displaced 2.5 cm away from the cautery (Figure 13). The different generations of the Vasoview System offer an assortment of dividing/ligating technologies, including bipolar scissors, bipolar ligating bisector tool and direct current cut-and-seal ligating graspers. [One should refer to the specific Instructions for Use (IFU) for each device to learn the particulars of each]. Importantly, minimal stretching or torqueing of the pedicle should be applied when addressing the branches so as to not incur intimal injury to the RA. Once all the side branches are divided from the RA, the cradle is slid gently up and down the pedicle to confirm completeness of debranching (Figure 14).

A 1 cm incision is made externally at the proximal end of the harvest tunnel, 1 cm distal to the antecubital crease. Prior to making the incision, its appropriate location is verified by pushing down on the overlying skin while visualizing within the tunnel with the scope. After the incision is made, a hemostat is used to retrieve the RA pedicle (Figure 15), bring it to skin level, ligate the proximal stump, and divide the radial graft from the stump. The endoscope is then use to withdraw the RA from the harvest tunnel using the cradle, and the distal end is similarly ligated and divided. The radial graft is then cannulated as previously described, and flushed with solution. To ensure hemostasis of the tunnel, the endoscope is reintroduced, the tourniquet is released, and the tunnel is inspected for potential bleeders that are then addressed. The incisions are then closed, sterile dressings are applied, and an ace bandage loosely wrapped around the forearm.
CommentsOther Section
The RA has been assuming an increasingly prominent role in arterial revascularization, often being used when additional arterial conduits are desired in conjunction with the internal mammary arteries. The surgical armamentarium for harvesting is multifold, including (I) either an open or endoscopic operative approach; (II) alternative dissection planes and (III) alternative methods of handling the RA branches. In this article we present both our open and endoscopic approaches. Although much work still needs to be done to fully elucidate which approaches or techniques—if any—are superior, the most important universal dictum is to pay great respect to the RA’s propensity for vasospasm. A “no touch” technique will ensure the optimal quality and longevity of the RA conduit, whatever harvesting methodology is chosen.
AcknowledgementsOther Section
We would like to thank Maquet Getinge Group for providing photographs and illustrations for the endoscopic harvesting portion of the manuscript.
Disclosure: The authors declare no conflict of interest.
ReferencesOther Section
- Deb S, Cohen EA, Singh SK, et al. Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: results from RAPS (Radial Artery Patency Study). J Am Coll Cardiol 2012;60:28-35. [PubMed]
- Reyes AT, Frame R, Brodman RF. Technique for harvesting the radial artery as a coronary artery bypass graft. Ann Thorac Surg 1995;59:118-26. [PubMed]
- Conklin LD, Ferguson ER, Reardon MJ. The technical aspects of radial artery harvesting. Tex Heart Inst J 2001;28:129-31. [PubMed]
- Beldner S, Zlotolow DA, Melone CP Jr, et al. Anatomy of the lateral antebrachial cutaneous and superficial radial nerves in the forearm: a cadaveric and clinical study. J Hand Surg Am 2005;30:1226-30. [PubMed]
- Taggart DP, Mathur MN, Ahmad I. Skeletonization of the radial artery: advantages over the pedicled technique. Ann Thorac Surg 2001;72:298-9. [PubMed]
- Sajja LR, Mannam G, Sompalli S. Extrafascially harvested radial artery in CABG: technique of harvest, complications, and mid-term angiographic patency. J Card Surg 2005;20:440-8. [PubMed]
- Strauch B. YH, Chen ZW, Liebling R. Forearm Region. Atlas of Microvascular Surgery: Anatomy and Operative Approaches. New York: Thieme Medical. 1993:44-83.
- Marzban M, Arya R, Mandegar MH, et al. Sharp dissection versus electrocautery for radial artery harvesting. Tex Heart Inst J 2006;33:9-13. [PubMed]





