Bilateral internal mammary arteries: evidence and technical considerations
“Numerous surgical groups around the world are reaching the conclusion that complete revascularization with multiple arterial grafts can be reliably achieved without cardiopulmonary bypass and often without aortic manipulation. Whenever feasible, this surgical strategy is most likely to yield excellent short- and long-term results for coronary artery bypass surgery patients.”
John Puskas MD, 2007
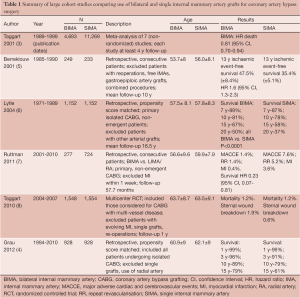
The survival benefit provided by a left internal mammary artery (LIMA) graft to the left anterior descending (LAD) artery has been acknowledged by clinicians for several decades (1). Appropriately, the LIMA to LAD graft forms the backbone of modern day coronary artery surgery practice. Large observational studies have suggested a further survival benefit by grafting a second internal mammary artery (IMA) to the left coronary system (2-4) (all studies summarized in Table 1). To date, however, there have been no randomized trials (reporting beyond 1 year of follow-up) comparing the use of a second IMA with additional saphenous vein grafts. The recently published ESC/EACTS Guidelines for Coronary Artery Revascularisation (9) have recommended the use of a LIMA to the LAD and arterial grafts to the non-LAD system in patients with a reasonable life expectancy, with minimization of aortic manipulation where possible. The use of bilateral internal mammary arteries (BIMA) makes the adherence to these recommendations easier. However, the inclusion of a second internal mammary artery in the grafting regimen of the majority of surgeons is disappointingly low [4.1% of all coronary artery bypass grafts (CABG) in the US in 2009 (10), 12% in Europe (11-13) and 12.6% in Australia between 2004-2006] (14).
Full table
There are several compelling arguments for the use of skeletonized BIMA in coronary artery bypass surgery. These include a survival benefit (2-4,6,7,15), obtaining two conduits from a single sternotomy therefore reducing morbidity from other harvest sites, and where possible, providing two in-flows to the heart without having to perform a proximal aortic anastomosis. However it has been difficult to convince the vast majority of surgeons performing coronary artery bypass surgery of the benefit of the extra effort required to harvest and use a second IMA. Reasons put forward by surgeons for not using a second internal mammary include a “lack of evidence” for their use, increased sternal morbidity, increased operative time, and that elderly patients should only receive vein grafts as they will not obtain the survival benefit conferred in younger patients. In the following paragraphs we will attempt to provide evidence-based counter arguments to these assertions.
Evidence for the efficacy of the use of bilateral, rather than single, mammary artery grafting comes from large observational studies. The Cleveland Clinic has published observational studies that has established the superiority of both single (1) and later double internal mammary artery grafts (2,6) over saphenous vein grafts. A Lancet meta-analysis from 2001 by Taggart and colleagues, including over 15,000 patients, demonstrated the survival benefits of bilateral mammary artery grafting over single mammary artery grafting as further evidence of the benefits offered by the use of more than one internal mammary artery (3). Since the publication of this meta-analysis there have been numerous institutional series (4,7,15) demonstrating the survival benefit of a second mammary artery. The 1-year results of the randomized Arterial Revascularization Therapies Study (ARTS) have recently been published, and demonstrated similar safety between the use of single and bilateral IMA (other than a small increase in sternal wound dehiscence, which is described below). The medium and long-term data from this trial is eagerly awaited (8).
The rate of sternal dehiscence is increased with use of BIMA compared to a single IMA. The ARTS trial reported a 0.6% rate of sternal wound reconstruction for patients who have a single IMA harvested compared with 1.9% for those having BIMA. Despite this, there was no difference in length of hospital stay or quality of life at 12 months. Those requiring sternal wound reconstruction were more likely to be diabetic compared with those who did not (8). Female sex, obesity, and diabetes are risk factors for sternal wound complications (16,17) while HbA1c has been demonstrated as a positive predictor (18). Sternal morbidity is reduced if the IMAs are harvested in a skeletonized fashion and care is taken not to injure the veins and muscle behind the sternum (19,20), thus improving sternal perfusion (21,22). The small increase in the short term risk of sternal wound infection must be balanced with increased graft patency and survival in the medium to long term.
Procedural time is necessarily increased when bilateral IMAs are used as they must be harvested sequentially, rather than simultaneously as is the case with vein and radial artery grafts. This is not conducive to (often) time-poor surgeons pursuing this technique. However, as surgeons become more proficient, the procedural time decreases. Taggart and colleagues demonstrated a 23 minute increase in procedural time for the inclusion of a second internal mammary artery in the grafting strategy (8). Our philosophy is that in the short term, the patient is saved from an extra incision for alternative conduit harvest, but the increased survival benefit makes the extra time and effort worthwhile.
Elderly patients can benefit from arterial grafts. Patients >75 years had better cardiac event-free survival when two arterial grafts (compared with only one) are used in one randomized trial, and another observational study (23,24). The use of BIMA grafts in the elderly means that more often than not, the entire procedure can be conducted via a sternotomy, sparing the legs and arms to facilitate more rapid mobilization and return to normal function by reducing extremity harvest site wound morbidity. Elderly patients often have sub-optimal venous conduit due to varicosities and calcification (25), which may be prone to early occlusion. The IMAs, in contrast, are often large with slightly thickened walls, facilitating easy harvest and manipulation during coronary bypass surgery. The use of vein grafts usually requires aortic in-flow (although we do use IMA/vein composite grafts) and that must be obtained using some degree of aortic manipulation. This exposes the patient to the inherent risk of athero-emboli and (albeit small) risk of iatrogenic type A dissection. We use a Maquet “Heart-String” device should an aorto-coronary anastomosis be absolutely necessary.
Technical considerations and surgical technique
All patients have bilateral carotid and subclavian artery duplex scans to assess concomitant peripheral vascular disease and the suitability of the IMAs for in-flow. Occasionally, patients require subclavian stenting prior to surgery. Patients have a low-dose Milrinone infusion (0.2 μg/kg/min) commenced at the beginning of the procedure to assist with vasodilatation of the arterial grafts.
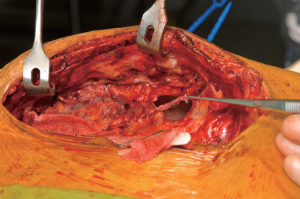
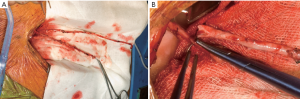
Both internal mammary arteries are harvested using a completely skeletonized technique (Figure 1). This minimizes chest wall trauma, achieves maximum length, and makes constructing the “T” graft and performing subsequent sequential grafting easier. The IMA is exposed by pushing the pleura laterally using either blunt and sharp diathermy dissection or a blunt dissection with a small gauze swab. Where possible, the pleural curtain is left intact. If this is not possible then the majority of the pleura is left intact to prevent pleural adhesions and the lungs from becoming adherent to the posterior sternal table in the event that reoperation is required.
Diathermy is used on low-power (coagulation 20 or less) with long, fine-tipped forceps (Dietrich’s). The endo-thoracic fascia is carefully incised directly below and along the length of the IMA. Some surgeons may prefer to use the harmonic scalpel for mammary harvesting. Either way, the low energy delivery and minimal handling technique is desirable for the prevention of injury including avulsion of small branches, dissection and arterial spasm. The medial internal mammary vein is identified at the subclavian vein and, if necessary, divided after double ligation on both sides. This often improves the final length and lie of the IMA and makes proximal harvest safer and easier. It is important to expose the superior aspect of the subclavian vein proximally and sweep this down to provide adequate exposure of the proximal IMA. The soft tissue between the undersurface of the strap (sternothyroid) muscles and the thymic remnant is divided to expose the subclavian vein. This allows the identification and division of the most proximal branches of the IMA, thus preventing proximal steal syndrome. Staying close to the IMA at the proximal extent may also reduce the incidence of inadvertent injury to the phrenic nerve. Harvesting both internal mammary arteries obviously exposes the patient to the rare, but devastating complications of bilateral phrenic nerve injury.
The IMA is then harvested using a combination of blunt and sharp diathermy dissection dividing branches with scissors after clipping both sides. Reducing the ventilatory tidal volumes or a brief period of apnea when harvesting the proximal IMA can make harvesting easier, quicker and safer. The IMA is harvested proximally from above the left subclavian vein to its bifurcation, while ensuring that all major branches are divided. The IMA is divided distally after systemic heparinization and then sprayed liberally with papaverine solution before being wrapped in a warm, papaverine soaked gauze. If the RIMA is to be used as the side arm for a “T” graft, then it is divided proximally and distally after heparinization. The proximal end is prepared for the graft with a short slit made with fine scissors on the under-surface of the vessel which is then soaked in heparinized blood containing 5 mg of Verapamil until use.
Graft combinations
Both IMAs are left un-italicise and a final assessment is made before committing to the final grafting strategy. The heart and target coronary arteries are inspected and the IMA caliber and length is assessed. For patients requiring left-sided grafts we use BIMA, either in situ (using RIMA to LAD; LIMA to lateral wall) or as a “T” graft (LIMA to LAD; RIMA to lateral wall). In our experience there is usually insufficient length for the RIMA to reach the inferior wall target vessels as a free graft from the in situ LIMA. If patients require all three territories grafted we prefer using a RIMA/radial extension graft (radial artery also skeletonized) via the transverse sinus to the lateral and inferior walls and a LIMA to the LAD (and diagonal if required). An alternative to this strategy is using a LIMA/RIMA “T” graft to the left system with a RIMA/radial extension graft to the inferior wall. In the rare event that the radial artery is not usable, a reversed long saphenous vein graft may be used to extend the RIMA and brought either over the aorta to the lateral wall or down the right side of the heart to the inferior wall (Figure 2). The use of two IMAs for all patients is obviously ideal, but we do use a LIMA/radial “T” graft for very elderly or morbidly obese patients requiring three vessel revascularizations, thus reducing operative times and potential morbidity.
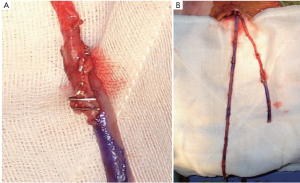
Preparation of the composite LIMA/RIMA “T” graft and composite grafts
The IMAs must be brought into the pericardium without being damaged or kinked. The pericardium is kept intact and the thymus is dissected free from below, along the length of the aortic arch (left) and the superior vena cava (right) to provide a reliable and reproducible passage for the IMA to be brought into the pericardium (Figure 3).
The pulmonary valve is the ideal position for the “T” graft. It provides a consistent point of reference when constructing the graft and importantly it maintains the straight lie of the LIMA to LAD graft, thus preventing kinking. The LIMA is gently pulled out to length within the retrothymic tunnel (Figure 3). Care must be taken to avoid excessive traction resulting in the avulsion of clips from arterial side-branches, which may cause bleeding or a localized dissection resulting in a loss of in-flow to the graft. It is important to carefully check the lie of the LIMA before the position of the “T” graft anastomosis is chosen. If there is too much length behind the thymus then there is a risk that the in-flow portion of the LIMA graft may kink.
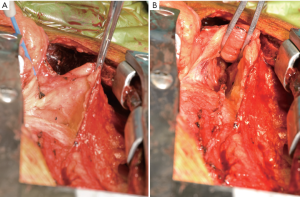
If an in situ RIMA is to be used then the thymic fat is mobilized from the pericardium over the ascending aorta and over the superior vena cava. A generous tunnel is made to bring the RIMA into the pericardium. It is important to divide the pericardium vertically at the superior vena cava and extend this inferiorly to mobilize the pericardium at the cavo-atrial junction to remove any risk of the RIMA kinking over the pericardium. This is especially important when passing a RIMA/radial composite graft via the transverse sinus to the left-side of the heart, or via the right-side of the heart to the inferior wall.
A cradle is made with a medium dry sponge and a folded wet sponge, suspended over the heart with artery clips. This allows optimal conditions for the careful fashioning of the “T” and composite grafts (Figure 4). All arterial anastomoses are performed using Ethicon® “flat-pack” 60 cm 7/0 Prolene with an 8.0 mm needle. This allows maximum flexibility for parachuting anastomoses with the benefit of minimal “memory” in the suture and the advantages of a smaller, sharper needle without the difficulty associated with 8/0 Prolene (ease of fracture, tangles etc.). The “T” anastomosis is performed on the superior aspect of the LIMA with the flow controlled proximally with a soft bull-dog clamp. The anastomosis is tightened and tied down after release of the bull-dog clamp. This allows one to maximize anastomosis tension in order to prevent leaks without causing “purse-stringing”. The composite graft anastomoses are performed in a beveled end-to-end fashion to deal with any size mismatch. In the event that a vein graft is used to extend an IMA (usually significant size mismatch) then a side-to-side anastomosis is performed with the ends of the grafts double-clipped with a medium liga-clip. If there is any residual vasospasm the grafts are flushed with heparinized/verapamil blood via the radial artery and the outside spayed with papaverine solution. Care is taken to release any soft-tissue bands across the arterial grafts with a number 11 scalpel blade.
Considerations and concerns when using “T” grafts
There is some concern when using “T” grafts of the potential implications of losing the LIMA to LAD component of the graft as a consequence of native circulation competitive flow. This obviously removes the significant benefit that a patent LIMA to LAD affords a patient in terms of survival and freedom from major adverse and cardiovascular events (MACCE). Early graft failure is often related to technical mistakes when constructing the “T” graft or the distal anastomosis and care must be taken when performing this anastomosis. Later failure is often related to competitive flow, either in the LAD or from the other target arteries, or to intimal hyperplasia or progression of native coronary artery disease. This reinforces the need to objectively assess lesions prior to making decisions on grafting strategies. Nonetheless, there is both randomized (23,26) and observational evidence (27-30) that composite grafting does not compromise graft patency or survival.
Another concern expressed by some surgeons is that a single IMA in-flow is insufficient for total cardiac revascularization. However, the internal mammary artery is usually significantly larger than the left main coronary artery, and this supplies the vast majority of the heart. The LIMA also has the capacity to dilate according to demand and this occurs shortly after grafting and further improves over time (31,32).
BIMA facilitates anaortic off-pump coronary artery bypass (OPCAB)
The coronary grafting strategy we have adopted is one of total arterial, anaortic, off-pump surgery utilizing BIMA in the vast majority of patients (33). The use of bilateral in situ or composite graft IMAs has made this approach technically straightforward. The most important benefit of avoiding aortic manipulation is the significant improvement in the rate of stroke, 0.29% in a recent meta-analysis (34,35), to a rate that is comparable to that of PCI (36). Indeed, BIMA grafting, even when performed on-pump, has been shown to decrease the rate of stroke by decreasing the rate of aortic manipulation (7).
Conclusions
A second internal mammary artery, and indeed a second arterial graft, has been shown to provide a survival benefit and freedom from MACCE in patients undergoing coronary artery bypass surgery. Using BIMA and total arterial grafting is potentially more time consuming and technically more difficult, but this is surely justified by improved patient outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [PubMed]
- Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg 1999;117:855-72. [PubMed]
- Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001;358:870-5. [PubMed]
- Grau JB, Ferrari G, Mak AW, et al. Propensity matched analysis of bilateral internal mammary artery versus single left internal mammary artery grafting at 17-year follow-up: validation of a contemporary surgical experience. Eur J Cardiothorac Surg 2012;41:770-5; discussion 776. [PubMed]
- Berreklouw E, Rademakers PP, Koster JM, et al. Better ischemic event-free survival after two internal thoracic artery grafts: 13 years of follow-up. Ann Thorac Surg 2001;72:1535-41; discussion 2012-4. [PubMed]
- Lytle BW, Blackstone EH, Sabik JF, et al. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg 2004;78:2005-12; discussion 2012-4.
- Ruttmann E, Fischler N, Sakic A, et al. Second internal thoracic artery versus radial artery in coronary artery bypass grafting: a long-term, propensity score-matched follow-up study. Circulation 2011;124:1321-9. [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J 2010;31:2470-81. [PubMed]
- Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55. [PubMed]
- ElBardissi AW, Aranki SF, Sheng S, et al. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg 2012;143:273-81. [PubMed]
- Kappetein AP. Bilateral mammary artery vs. single mammary artery grafting: promising early results: but will the match finish with enough players? Eur Heart J 2010;31:2444-6. [PubMed]
- Kappetein AP, Dawkins KD, Mohr FW, et al. Current percutaneous coronary intervention and coronary artery bypass grafting practices for three-vessel and left main coronary artery disease. Insights from the SYNTAX run-in phase. Eur J Cardiothorac Surg 2006;29:486-91. [PubMed]
- Taggart DP. Bilateral internal mammary arteries: a very important missing trick for coronary artery bypass grafting. Eur J Cardiothorac Surg 2012;41:776-7. [PubMed]
- Yan BP, Clark DJ, Buxton B, et al. Clinical characteristics and early mortality of patients undergoing coronary artery bypass grafting compared to percutaneous coronary intervention: insights from the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) and the Melbourne Interventional Group (MIG) Registries. Heart Lung Circ 2009;18:184-90. [PubMed]
- Endo M, Nishida H, Tomizawa Y, et al. Benefit of bilateral over single internal mammary artery grafts for multiple coronary artery bypass grafting. Circulation 2001;104:2164-70. [PubMed]
- Hirose H, Amano A, Takanashi S, et al. Skeletonized bilateral internal mammary artery grafting for patients with diabetes. Interact Cardiovasc Thorac Surg 2003;2:287-92. [PubMed]
- Saso S, James D, Vecht JA, et al. Effect of skeletonization of the internal thoracic artery for coronary revascularization on the incidence of sternal wound infection. Ann Thorac Surg 2010;89:661-70. [PubMed]
- Halkos ME, Thourani VH, Lattouf OM, et al. Preoperative hemoglobin a1c predicts sternal wound infection after coronary artery bypass surgery with bilateral versus single internal thoracic artery grafts. Innovations (Phila) 2008;3:131-8. [PubMed]
- Calafiore AM, Vitolla G, Iaco AL, et al. Bilateral internal mammary artery grafting: midterm results of pedicled versus skeletonized conduits. Ann Thorac Surg 1999;67:1637-42. [PubMed]
- Peterson MD, Borger MA, Rao V, et al. Skeletonization of bilateral internal thoracic artery grafts lowers the risk of sternal infection in patients with diabetes. J Thorac Cardiovasc Surg 2003;126:1314-9. [PubMed]
- Boodhwani M, Lam BK, Nathan HJ, et al. Skeletonized internal thoracic artery harvest reduces pain and dysesthesia and improves sternal perfusion after coronary artery bypass surgery: a randomized, double-blind, within-patient comparison. Circulation 2006;114:766-73. [PubMed]
- Parish MA, Asai T, Grossi EA, et al. The effects of different techniques of internal mammary artery harvesting on sternal blood flow. J Thorac Cardiovasc Surg 1992;104:1303-7. [PubMed]
- Nasso G, Coppola R, Bonifazi R, et al. Arterial revascularization in primary coronary artery bypass grafting: Direct comparison of 4 strategies--results of the Stand-in-Y Mammary Study. J Thorac Cardiovasc Surg 2009;137:1093-100. [PubMed]
- Jones JW, Schmidt SE, Miller CC 3rd, et al. Bilateral internal thoracic artery operations in the elderly. J Cardiovasc Surg (Torino) 2000;41:165-70. [PubMed]
- Evans CJ, Fowkes FG, Ruckley CV, et al. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health 1999;53:149-53. [PubMed]
- Muneretto C, Bisleri G, Negri A, et al. Total arterial myocardial revascularization with composite grafts improves results of coronary surgery in elderly: a prospective randomized comparison with conventional coronary artery bypass surgery. Circulation 2003;108:II29-33. [PubMed]
- Barner HB, Bailey M, Guthrie TJ, et al. Radial artery free and T graft patency as coronary artery bypass conduit over a 15-year period. Circulation 2012;126:S140-4. [PubMed]
- Lemma M, Mangini A, Gelpi G, et al. Is it better to use the radial artery as a composite graft? Clinical and angiographic results of aorto-coronary versus Y-graft. Eur J Cardiothorac Surg 2004;26:110-7. [PubMed]
- Lev-Ran O, Paz Y, Pevni D, et al. Bilateral internal thoracic artery grafting: midterm results of composite versus in situ crossover graft. Ann Thorac Surg 2002;74:704-10; discussion 710-1. [PubMed]
- Maniar HS, Barner HB, Bailey MS, et al. Radial artery patency: are aortocoronary conduits superior to composite grafting? Ann Thorac Surg 2003;76:1498-503; discussion 1503-4. [PubMed]
- Gaudino M, Di Mauro M, Iacò AL, et al. Immediate flow reserve of Y thoracic artery grafts: an intraoperative flowmetric study. J Thorac Cardiovasc Surg 2003;126:1076-9. [PubMed]
- Glineur D, Boodhwani M, Poncelet A, et al. Comparison of fractional flow reserve of composite Y-grafts with saphenous vein or right internal thoracic arteries. J Thorac Cardiovasc Surg 2010;140:639-45. [PubMed]
- Vallely MP, Yan TD, Edelman JJ, et al. Anaortic, total-arterial, off-pump coronary artery bypass surgery: how to do it. Heart Lung Circ 2010;19:555-60. [PubMed]
- Edelman JJ, Yan TD, Bannon PG, et al. Coronary artery bypass grafting with and without manipulation of the ascending aorta--a meta-analysis. Heart Lung Circ 2011;20:318-24. [PubMed]
- Edelman JJ, Yan TD, Vallely MP. Anaortic off-pump coronary artery bypass grafting: The criterion standard for minimization of neurologic injury. J Thorac Cardiovasc Surg 2012;143:251-2; author reply 252. [PubMed]
- Edelman JJ, Yan TD, Padang R, et al. Off-pump coronary artery bypass surgery versus percutaneous coronary intervention: a meta-analysis of randomized and nonrandomized studies. Ann Thorac Surg 2010;90:1384-90. [PubMed]