Frozen elephant trunk surgery—the Bologna’s experience
Introduction
The continuous search for improved techniques in the treatment of patients with extensive disease of the thoracic aorta represents a formidable challenge for the cardiovascular surgeon. The encroachment of thoracic aortic endovascular repair into the arch segment has promoted the development of different hybrid approaches with the frozen elephant trunk (FET) strategy, which includes classic arch replacement and antegrade stenting of the descending thoracic aorta, emerging as a valuable technique (1-7). In the present study, we report our experience with the FET and discuss the indications and outcomes of this approach.
Patients and methods
Patients
Between January 2007 and July 2012, 122 patients underwent extensive thoracic aorta surgery using the FET approach with an E-vita open prosthesis (JOTEC® GmbH, Germany). Pre-, intra- and post-operative data were obtained using a prospective database supplemented by a chart review. Institutional review board approval was obtained for data collection and analysis, with a waiver for individual consent.
Surgical technique
In the present series, the E-vita Open and E-vita Open Plus prostheses were used in all cases for the FET construction. The 2nd generation E-vita Open Plus prosthesis differs from the 1st generation E-vita Open as it has a proximal zero-porosity Dacron portion, making it suitable for aortic arch replacement.
Brain protection was achieved with bilateral selective antegrade cerebral perfusion (SACP; flow rate: 10 mL/kg/min; perfusion pressure: 40-70 mmHg) and moderate hypothermia (26 °C) in all cases. Monitoring for SACP included bilateral radial artery pressure lines, nasopharyngeal and bladder temperatures, and regional oxygen saturation in the frontal lobes by near-infrared spectroscopy (8). Spinal cord fluid drainage was routinely used in the last years of the study period.
Our technique for arch replacement using the FET technique has been described previously (9). In brief, all procedures were performed using a median sternotomy. After systemic heparinization, a guidewire was inserted through the femoral artery and advanced to the ascending aorta. In patients with aortic dissection, trans-esophageal echocardiography confirmed the correct positioning of the guide-wire in the true lumen.
For cardiopulmonary bypass (CPB), the aorta, axillary, innominate and femoral arteries were used as sites for arterial inflow while the right atrium or bi-caval cannulation ensured venous drainage. A left ventricle drain was inserted through the right superior pulmonary vein. Myocardial protection was achieved using crystalloid cold cardioplegia (Custodiol, Koehler Chemie, Alsbach-Haenlein, Germany). When feasible, proximal repairs were initiated during the cooling phase. Subsequently, circulatory arrest was instituted at a nasopharyngeal temperature of 26 °C, and the aortic arch was opened and bilateral SACP was established. Our perfusion strategy most frequently contemplated right axillary or innominate artery cannulation for perfusion of the right hemisphere and endoluminal cannulation of the left common carotid artery for perfusion of the left hemisphere. The left subclavian artery was often cannulated to improve proximal spinal cord and left vertebral system perfusion. The arch was completely resected and the proximal descending aorta was prepared for the distal anastomosis. For selection of the graft size, a 10-20% oversizing of the measurement of the distal landing zone was used for degenerative aneurysms. Oversizing was avoided in dissected patients. Sizing was based on the diameters of the descending aorta for acute dissections as well as the diameter of the true lumen for chronic dissections, as measured preoperatively using computed tomography (CT) scan or intra-operatively using standard sizers. In patients with acute or chronic dissection, obliteration of the false lumen was obtained using external Teflon felt fixed with four internal pledgetted U-stitches. The stent-graft system was then introduced over the guide-wire and deployed in the descending thoracic aorta. The incorporated Dacron graft was pulled back and sutured to the previously prepared descending aorta using a 2-0 polypropylene running suture. After ten minutes of lower body reperfusion, the arch vessels were re-implanted using the E-vita Open Plus graft or a separate graft when the E-vita Open was employed. The proximal anastomosis usually completed the aortic repair.
Statistical analysis
Continuous variables were expressed as the mean ± SD and were analyzed using the unpaired 2-tailed t-test. The categorical variables were presented as percentages and were analyzed with the χ2 test or Fisher exact test. A 2-tailed P value less than 0.05 was considered statistically significant. All the pre- or intra-operative variables which achieved P values less than 0.05 in the univariate analysis were examined using multivariate analysis by stepwise logistic regression to evaluate independent risk factors for adverse outcome, a composite outcome variable including death ± permanent neurological dysfunction (PND: stroke or coma) ± spinal cord injury (SCI: paraplegia/paraparesis).
Patients were followed in the outpatient clinic, and by CT/magnetic resonance imaging reviews and telephone calls. For the surviving patients, survival curves were estimated at 1- and 3-year using the Kaplan-Meier method. Independent predictors of 3-year mortality were determined using Cox proportional hazards analysis. Statistical analysis was carried out using SPSS 20.0.
Results
Patient characteristics
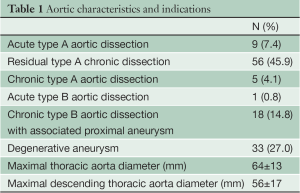
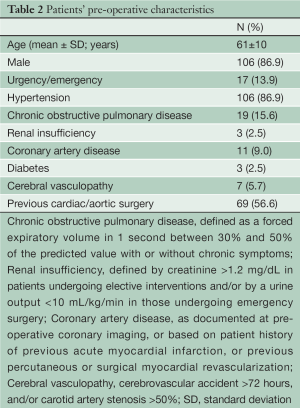
Between January 2007 and July 2012, 122 patients (male: 86.9%; mean age: 61 years) underwent extensive thoracic aorta surgery using the FET approach with an E-vita open prosthesis. The most frequent indications for surgery included residual type A chronic dissection (45.9%), extensive degenerative aneurysm of the thoracic aorta or saccular aneurysm of the distal aortic arch (27%), and type A acute aortic dissection (7.4%) (Table 1). In type A acute aortic dissection patients, the indications for the FET technique included an entry tear located in the distal aortic arch (n=4), a distal aortic arch rupture (n=1), a dissecting lesion limited to the proximal descending thoracic aorta (n=1), and young age (n=3). The most relevant preoperative comorbidities are illustrated in Table 2.
Full table
Full table
Operative data
During CPB, an antegrade aortic flow was obtained in 108 (88.5%) patients using the right axillary artery (n=69), the innominate artery (n=26), and the ascending aorta (n=13) as sites for arterial cannulation. The arch was completely replaced in 118 (96.7%) patients, while in 4 patients, the arch was opened longitudinally to allow the deployment and fixation of the hybrid prosthesis, and was then re-sutured without resection. The most frequently associated procedures involved the aortic valve and root (76.6%). Operative times are shown in Table 3.
Full table
Hospital outcomes
Overall, 21 (17.2%) deaths occurred, 16 (15.2%) in elective and 5 (29.4%) in urgent patients (P=0.1), respectively. The causes of death were aortic (n=1), multi-organ failure (n=14), neurologic (n=3), cardiac (n=2), and pancreatitis (n=1). As assessed by the surgeons, anesthesiologists and neurologists, PND (stroke or coma) occurred in 9 patents (7.4%), and SCI (paraplegia/paraparesis) in 11 (9.0%). Adverse outcome, a composite outcome variable for death ± PND ± SCI, occurred in 22.1% of cases. The other most relevant postoperative complications are shown in Table 4.
Full table
With multivariate logistic regression, CPB time emerged as the only independent predictive risk factor for an adverse outcome [odds ratio (OR), 1.007/min; 95% CI, 1.000-1.013; P=0.045].
Follow-up
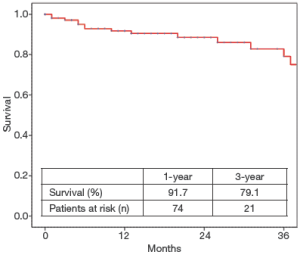
The follow-up was 100% complete. Thirteen deaths occurred during follow-up. For the surviving patients, 1- and 3-year freedom (%) from all-cause mortality was (91.7±2.8)% and (79.1±6.1)%, respectively (Figure 1).
Among all pre- and intra-operative variables, age (OR, 1.08; 95% CI, 1.00-1.15; P=0.034) and diabetes (OR, 7.5; 95% CI, 1.6-35.6) were identified as independent predictive risk factors for all-cause death at follow-up.
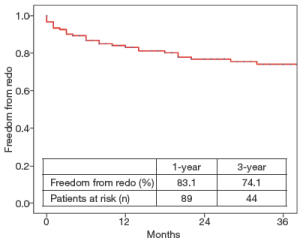
During the follow-up period, 29 aortic interventions were performed, 26 of which consisted in an endovascular distal aortic extension, 2 in an abdominal aorta aneurysm resection, and 1 in the resection of a sub-valvular aortic stenosis. Overall, 1- and 3-year freedom from re-intervention was (83.1±3.5)% and (74.1±4.3)%, respectively (Figure 2). As compared to other underlying aortic diseases, chronic dissection was associated with a higher rate of re-intervention (29.1% vs. 14.0%; P=0.06). Nevertheless, we were not able to identify the independent predictive risk factors for re-intervention at follow up. Overall, secondary aortic procedures were associated with a 100% technical success rate and 100% freedom from death and major complications.
Discussion
The FET approach has emerged as an interesting form of treatment for patients with extensive disease of the thoracic aorta, and its application has significantly increased over recent years.
At our institution, the FET technique was first performed in 2007. Since then, indications have been extended significantly and currently include a wide variety of diseases, ranging from degenerative aneurysms of the aortic arch to acute or chronic aortic dissections, type A or B (Figure 3).
During surgery for acute dissection, patients with complex arch tears involving the distal arch and/or proximal descending thoracic aorta certainly represent an interesting subset for FET application. In fact, this approach can simplify the surgical procedure, as the distal anastomosis can be easier and more safely performed at a more proximal level, while the incorporated stent-graft can accomplish the more demanding distal repair and cover the entry tear (10). Catastrophic conditions of distal malperfusion with compression of the true lumen at the descending thoracic aorta can also be approached using the FET technique, which, favoring false lumen obliteration and true lumen re-opening, may re-establish optimal end-organ perfusion and improve final outcomes. Studies from Hessen University (11) and the Cleveland Clinic (7) have shown how FET techniques (which are often associated with secondary endovascular procedures to optimize final results) can result in superior outcomes for this high-risk group of malperfused patients when appropriately employed in hybrid operative rooms by a multi-disciplinary team. In acute dissections, the FET technique, by favoring distal aortic remodeling, is also expected to improve long-term outcomes by reducing the number of late aortic events, re-interventions and mortality (12,13). If this proves to be true, the prognosis of patients surviving primary interventions for DeBakey Type I acute dissection will dramatically improve. For the time being, despite encouraging data showing improved radiological results (remodeling) and trends towards fewer re-interventions in FET patients (3,5,14), a substantial survival benefit of the FET versus more conservative techniques has yet to be demonstrated, and more robust data are necessary to standardize new paradigms of treatment in patients with acute dissection. Hence, while experienced surgeons and centers are called upon to thoughtfully explore new forms of treatment, dissection-related as well as non-dissection-related (age, connective tissue disease, surgical experience) factors remain key determinants in deciding how aggressively to address the dissected descending thoracic aorta in DeBakey type I acute dissection patients.
During surgery for chronic dissection, the conventional surgical approach would consider an initial open surgical replacement of the aortic arch using the classic elephant trunk technique, followed by an open repair of the aneurysmal descending or thoraco-abdominal aorta (15). Although this elegant approach has contributed greatly to aortic surgery and has allowed patients to be treated with satisfactory results (16), it is still associated with some limitations, including increased mortality due to two major aortic procedures, interval mortality, and failure to complete aortic repair. These shortcomings can be partially attenuated by the alternative FET technique.
The conventional elephant trunk techniques and FET represent opposite approaches to treatment of the dissected descending aorta. At primary intervention, the FET technique aims to depressurize and induce thrombosis of the peri-stent dilated false lumen (Figure 4). Conversely, the conventional elephant trunk technique, by requiring a wide distal surgical intimal fenestration, results in high pressurization of the false lumen and little chance for late false lumen thrombosis (9). The results from the most important series (17-21) using the conventional elephant trunk approach have shown that up to 25% of patients die during the interval between the two interventions, mostly due to aortic rupture. Taking this into account, the FET technique may be protective against interval mortality. In our experience, distal malperfusion secondary to the surgical obliteration of the false lumen has not been a concern in FET patients, most likely due to careful patient selection. After careful assessment of the aortic anatomy at pre-operative imaging, it was determined that true and false lumina at the distal descending and abdominal aorta almost invariably communicate by numerous distal re-entries, which ultimately guarantee adequate end-organ perfusion. In chronic dissection, the use of stent-grafts and FET technique is also questionable because it does not constitute a definitive repair. The thickened fibrotic lamella will not allow the stent-graft to obliterate the false lumen, and the ever-present distal re-entries will not permit depressurization and thrombosis of the distal false lumen. This remains true, and ultimately translates into the necessary meticulous clinical and imaging surveillance of FET patients at risk for further distal re-interventions. In our experience, 26 patients underwent secondary endovascular extension to cover the re-entry tears at the distal descending thoracic aorta. However, it is worth noting that: (I) the stented elephant trunk represents an excellent proximal landing zone for secondary stent-graft deployment, (II) outcomes after secondary endovascular procedures were excellent with a 100% technical success rate, and no deaths or major complications, and (III) this approach, which involves patient surveillance and prompt endovascular extensions to optimize thrombosis of the false lumen, appears to effectively control aneurysmal degeneration of the dissected descending thoracic aorta. Furthermore, compared to the proximal descending aorta, the dissected abdominal aorta shows more stable behavior with infrequent and slower dilatation during follow-up despite the common presence of numerous re-entry tears (Figure 4A). In the last year, we offered an abdominal aorta replacement with de-branching of the visceral vessels followed by conclusive stent-grafting of the residual aortic segments to those few patients who have shown progressive aneurysmal degeneration of the distal descending thoracic and abdominal aorta (Figure 4B). Based on the recent “collateral network” notion (22), which indicates that restoring good spinal cord perfusion is a time-dependent process, our staged abdominal de-branching procedures are expected to be associated with improved spinal cord and renal outcomes. Our initial results appear promising, but are certainly not sufficient to reach any definitive conclusion.
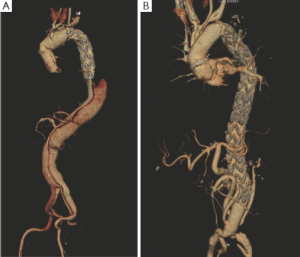
During surgery for degenerative aneurysms, the FET technique was found to be extremely effective in patients whose aneurysms were confined to the arch and proximal descending thoracic aorta. This technique was also effective in those presenting with saccular aneurysms of the mid-distal arch for which treatment with stent-grafting was deemed technically unsuccessful.
FET surgery is not completely free from complications. In line with reports by the International E-Vita open Registry (23), our hospital outcomes indicate higher mortality and brain injury rates for FET compared to that of more conservative management. It should be considered, however, that assessing the outcomes of the FET versus hemiarch or total arch with classic elephant trunk procedures is problematic, mainly due to different indications, the extent of the aortic disease, study intervals, and surgical risks that patients may have with these two distinct approaches (16,24). In our opinion, our adverse outcome rate of 22.1% reflects the complexity of our surgical population with a relevant proportion of patients presenting with extensive disease of the thoracic aorta, acute or chronic dissection, re-interventions, and associated procedures. The finding of CPB time as the only independent risk factor for adverse outcome in the present series supports this notion.
The main apparent concern regarding FET surgery is the increased risk of paraplegia. In the present series, 9.0% of patients had this catastrophic complication, which ranges between 0% and 21.7% in the literature (25,26). The potential pathogenic mechanisms for spinal cord injury after the FET procedure include circulatory arrest, coverage of the intercostal arteries, embolization, and postoperative periods of hypotension. To avoid these complications, we employed moderate hypothermia, total brain perfusion with perfusion of the left subclavian artery, lower body perfusion to reduce the duration of circulatory arrest, cerebro-spinal liquor drainage, and maintenance of postoperative stable hemodynamics with a mean arterial pressure greater than 80 mmHg. Nevertheless, some of our patients were still affected by paraplegia. Indeed, our limited data does not allow identification of those associated with altered postoperative occurrence of SCI, nor preoperative risk factors and intraoperative adjuncts for spinal cord protection. Methods of decreasing SCI occurrence may possibly involve the use of shorter stent-grafts, deeper levels of hypothermia, and faster anastomotic aortic devices. While the latter will not be available for some time, the former is likely to be associated with an increased need for distal re-intervention and deep hypothermia-related complications. The efficacy of different solutions should also be researched. Given the risk of paraplegia and the increased costs of the FET technique, prophylactic elephant trunks should remain “classic”.
In our experience, FET surgery allowed the treatment of complex patients who have extensive thoracic aortic diseases with satisfactory short- and mid-term results. In acute dissection patients, a strong argument favoring the FET technique includes complex arch tears and distal aortic malperfusion. Other advantages related to FET-induced aortic remodeling are promising but require confirmation by larger studies with longer follow-up. In chronic dissection patients, the FET technique may represent a valuable bridge therapy towards a staged hybrid reconstruction of the entire thoraco-abdominal aorta. While longer-term studies are needed in order to show the survival benefits of the FET technique versus other types of management, new strategies for SCI reduction should be researched.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Shrestha M, Pichlmaier M, Martens A, et al. Total aortic arch replacement with a novel four-branched frozen elephant trunk graft: first-in-man results. Eur J Cardiothorac Surg 2013;43:406-10. [PubMed]
- Hoffman A, Parker JA, Raweh A, et al. Restoration of the thoracic aorta in Type A dissection with hybrid prosthesis. Asian Cardiovasc Thorac Ann 2011;19:123-7. [PubMed]
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Multicenter early experience with extended aortic repair in acute aortic dissection: is simultaneous descending stent grafting justified? J Thorac Cardiovasc Surg 2010;140:S116-20; discussion S142-S146.
- Di Bartolomeo R, Di Marco L, Armaro A, et al. Treatment of complex disease of the thoracic aorta: the frozen elephant trunk technique with the E-vita open prosthesis. Eur J Cardiothorac Surg 2009;35:671-5; discussion 675-6. [PubMed]
- Sun L, Qi R, Zhu J, Liu Y, et al. Total arch replacement combined with stented elephant trunk implantation: a new “standard” therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [PubMed]
- Pochettino A, Brinkman WT, Moeller P, et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg 2009;88:482-9; discussion 489-90. [PubMed]
- Roselli EE, Rafael A, Soltesz EG, et al. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg 2013;145:S197-201. [PubMed]
- Di Eusanio M, Schepens M, Morshuis W, et al. Operations on the thoracic aorta and antegrade selective cerebral perfusion: our experience with 462 patients. Ital Heart J 2004;5:217-22. [PubMed]
- Di Eusanio M, Armaro A, Di Marco L, et al. Short- and midterm results after hybrid treatment of chronic aortic dissection with the frozen elephant trunk technique. Eur J Cardiothorac Surg 2011;40:875-80. [PubMed]
- Di Eusanio M, Petridis FD, Pacini D, et al. Facilitated aortic arch repair with the frozen elephant trunk technique. Eur J Cardiothorac Surg 2011;40:1261-2. [PubMed]
- Tsagakis K, Konorza T, Dohle DS, et al. Hybrid operating room concept for combined diagnostics, intervention and surgery in acute type A dissection. Eur J Cardiothorac Surg 2013;43:397-404. [PubMed]
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. [PubMed]
- Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955-64; discussion 1955-64.
- Uchida N, Katayama A, Tamura K, et al. Frozen elephant trunk technique and partial remodeling for acute type A aortic dissection. Eur J Cardiothorac Surg 2011;40:1066-71. [PubMed]
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [PubMed]
- Ius F, Hagl C, Haverich A, et al. Elephant trunk procedure 27 years after Borst: what remains and what is new? Eur J Cardiothorac Surg 2011;40:1-11. [PubMed]
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004;78:109-16; discussion 109-16. [PubMed]
- Safi HJ, Miller CC 3rd, Estrera AL, et al. Staged repair of extensive aortic aneurysms: morbidity and mortality in the elephant trunk technique. Circulation 2001;104:2938-42. [PubMed]
- Etz CD, Plestis KA, Kari FA, et al. Staged repair of thoracic and thoracoabdominal aortic aneurysms using the elephant trunk technique: a consecutive series of 215 first stage and 120 complete repairs. Eur J Cardiothorac Surg 2008;34:605-14; discussion 614-5. [PubMed]
- LeMaire SA, Carter SA, Coselli JS. The elephant trunk technique for staged repair of complex aneurysms of the entire thoracic aorta. Ann Thorac Surg 2006;81:1561-9; discussion 1569. [PubMed]
- Schepens MA, Dossche KM, Morshuis WJ, et al. The elephant trunk technique: operative results in 100 consecutive patients. Eur J Cardiothorac Surg 2002;21:276-81. [PubMed]
- Etz CD, Zoli S, Mueller CS, et al. Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg 2010;139:1464-72. [PubMed]
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Arch replacement and downstream stent grafting in complex aortic dissection: first results of an international registry. Eur J Cardiothorac Surg 2011;39:87-93; discussion 93-4. [PubMed]
- Schepens MA. Editorial comment: Will the elephant trunk become frozen? Eur J Cardiothorac Surg 2011;40:11-2. [PubMed]
- Hoffman A, Damberg AL, Schälte G, et al. Thoracic stent graft sizing for frozen elephant trunk repair in acute type A dissection. J Thorac Cardiovasc Surg 2013;145:964-9. [PubMed]
- Leontyev S, Borger MA, Etz CD, et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]