Rate of repair in minimally invasive mitral valve surgery
Minimally invasive surgery has been in use for 15 years (1). The presumed benefits of this approach include improved cosmesis, and also reduced post-operative pain, blood loss, hospital stay, and time to return to normal activity. Numerous studies have confirmed the safety of this approach and its excellent mid-term outcomes (2-6). For patients who require surgical intervention for degenerative mitral valve regurgitation, it is generally accepted that surgical mitral valve repair is the gold standard procedure because of the significantly better results compared with valve replacement (7-9). However, there is doubt that minimally invasive mitral valve surgery can achieve the same efficiency as a standard sternotomy, and that technical compromise, due to limited space, may lead to mitral valve replacement in patients for whom repair would otherwise clearly be the preferred therapy. We report here our experience with mitral valve repair in a consecutive series of patients operated on minimally invasively and referred for degenerative mitral regurgitation.
Material and methods
Study population
We retrospectively identified a total of 842 patients with degenerative mitral valve regurgitation operated on with a minimally invasive approach between September 2006 and December 2012. Six hundred and thirty six patients (75.5%) were male, and 206 (24.5%) female. At the beginning of our experience, only simple repair procedures were considered for this technique. With increased experience, more complex repair cases were managed with this approach. Currently, mitral valve repair for degenerative disease of the mitral valve, either isolated or combined with tricuspid valve disease, atrial fibrillation or atrial septal defect, is approached minimally invasively, with the exception of the calcified mitral annulus. This is because the fragile dedicated surgical instruments are not adequate to handle this specific situation.
Of the 842 patients identified, 688 (81.7%) had isolated posterior leaflet prolapse, 82 (9.7%) isolated anterior leaflet prolapse and 72 (8.6%) had bileaflet prolapse. Patient demographics, cardiac comorbidities, and other risk factors are summarized in Table 1. Mean patient age was 56.15±11.62 (range, 21 to 87) years. One hundred and eighteen patients (14%) were ≥70 years of age. Mean left ventricular ejection fraction was 64.8%±5.2%. Six hundred and eighty three patients (81.1%) were in New York Heart Association (NYHA) functional class I or II whereas 159 (18.9%) patients were in NYHA functional class III or IV. Preoperative mitral valve regurgitation was moderately severe in 125 patients (14.8%) and severe in the remaining 717 (85.2%).
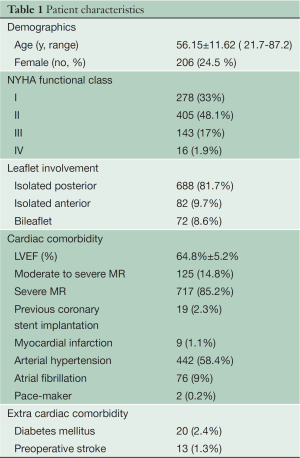
Full table
The composite outcome of 30-day MAE included the following: death, stroke, reoperation for valve dysfunction, urgent/emergency reoperation, perioperative myocardial infarction, renal failure, deep wound infection, new onset of permanent atrial fibrillation and prolonged mechanical ventilation (>24 hours).
Surgical technique
After induction of anesthesia, the patient was placed in the supine position, with slight elevation of the right hemithorax. Patients were ventilated either with a single or a double-lumen endotracheal tube, depending on the preference of the anesthesiologist. A single femoral venous cannula was routinely used except when the patient was above 1.9 m or weighed more than 100 kg. In these cases, an Edwards Fem-Flex II 16 FR cannula (Edwards Lifesciences, Irvine, CA) was placed by the anesthesiologist in the internal jugular vein under transoesophageal echocardiographic (TEE) guidance.
The anterior aspect of the right femoral vessels was exposed by using a 4 cm vertical right groin incision. A double and a single purse string suture (5-0 Prolene, Ethicon, Raleigh, NC) were placed respectively on the femoral artery and the femoral vein. After heparinization, the femoral vessels were cannulated with a Seldinger technique under TEE guidance. It is important to precisely place the tip of the venous cannula into the superior vena cava. Patients were cooled to 32 °C and vacuum assisted cardiopulmonary bypass (CPB) was used throughout the procedure.
A 3.5 to 5 cm right lateral working port was placed in the 4th intercostal space. The incision was placed below and lateral to the nipple in men, and in the submammary crease in women. CPB was instituted before opening the pleura. During the early part of our experience, a soft tissue retractor was utilized (Edwards Lifesciences, Santa Ana, CA, USA experience), followed by an “Alexis” wound protector (Applied Medical, Rancho Santa Margarita, CA, USA). No rib retractor was used. A 5 mm 30° angle endoscope was inserted through a port placed in the 4th intercostal space, posterior to the incision for the working port. The operation was carried out using the endoscopic image exclusively, without direct vision. The surgical field was flooded with carbon dioxide through the endoscope port throughout the procedure. After opening the pericardium away from the phrenic nerve, the pericardium was separated from the inferior vena cava and the inferior aspect of the left atrium.
A Chitwood aortic cross-clamp was inserted in the 3rd intercostal space. A cardioplegia needle (antegrade 7 FR, 30 cm, MAQUET cardiopulmonary AG, Germany) was placed in the ascending aorta, and then brought through the 2nd intercostal space to outside the thoracic cavity, and connected to the cardioplegia line. After aortic cross clamping, antegrade crystalloid Bretschneider solution was administered into the aortic root, and then repeated after 120 min if necessary. The operation was performed under moderate hypothermia (30-32 °C).
The left atrium was opened posterior to the interatrial groove and a left atrial blade was used to expose the mitral valve. Most often, folding of the inferior wall of the left atrium obstructed the view of part of the mitral valve and compromised the exposure. A 4-0 Prolene suture (Ethicon, Raleigh, NC, USA) was placed 1 cm behind the annulus, at five o’clock, and then through the pericardium beneath the inferior vena cava. Tying the knot invariably led to a satisfactory exposure of the mitral valve.
Specialized instruments introduced into the chest through the working port were used for tissue handling and suturing. Standard mitral valve repair techniques were used as described below. In all patients, a ring annuloplasty (Physio II, Edwards Lifesciences, Santa Ana, CA, USA) was performed. Once the repair was completed, the left atrium was closed, leaving a venting sucker through the mitral valve for de-airing. Suction was applied to the cardioplegic needle, and de-airing was guided by TEE. The aorta was then unclamped when no bubbles were visible in the cavities of the heart.
Valve repairs were performed using the “functional” approach technique conceptualized by Carpentier (10). Our typical approach was to remodel the posterior leaflet from the anterior to the posterior commissure to obtain a smooth and regular surface. It is essential that the free edge of the posterior leaflet is long enough to avoid a “curtain effect”. A triangular resection was used to correct any excess of tissue of the posterior leaflet in width. The indentations were almost always closed. Any residual prolapse of the posterior leaflet, or prolapse of the posterior leaflet without excess of tissue in width, was typically corrected with artificial chordae (4-0 polytetrafluoroethylene). The goal was that the posterior leaflet was positioned to remain in the inflow of the left ventricle. A prolapse of the anterior leaflet, when confirmed by surgical analysis was then corrected using either 4-0 polytetrafluoroethylene chordae or with “autologous” techniques such as chordal or papillary muscle shortening.
Limited triangular resection of the posterior leaflet was performed in 501 patients (59.5%) and a quadrangular resection in 19 patients (2.3%). In 460 patients (54.6%), artificial chordae, using 4-0 polytetrafluoroethylene (Gore-Tex, Flagstaff, Ariz), were implanted. A Carpentier-Edwards Physio II ring (Edwards Lifesciences, Irvine, CA, USA) was implanted in all cases. The ring size chosen was based on the surface area of the anterior mitral leaflet (ring 28 in 29 patients, ring 30 in 103, ring 32 in 224, ring 34 in 219, ring 36 in 128, ring 38 in 81, ring 40 in 52 patients). Intraoperative TEE was performed in all patients to check the quality of the repair.
Predischarge echocardiography
Two dimensional and Doppler transthoracic echocardiography examinations were performed before discharge in all patients. The presence of mitral regurgitation was assessed and its severity was evaluated semi-quantitatively by using previously validated color Doppler methods (11). The grade of regurgitation was classified as being 0, 0+ (barely detected regurgitation), mild, moderate and severe.
Data collection
Echocardiographic data, surgical findings, and repair techniques were prospectively collected and entered into our department database. Further information was obtained from patient records when necessary.
Results
Mitral valve repair was performed in 836 patients (99.3%). Six patients (0.7%) underwent mitral valve replacement. In four patients, residual mitral regurgitation after attempted repair prompted mitral valve replacement. One patient had severe annular calcification preventing valve repair, and satisfactory exposure of the mitral valve was impossible in another patient with kyphoscoliosis.
Adjunct procedures included atrial fibrillation therapy in 115 patients (13.6%) and tricuspid valve repair in 20 patients (2.4%). Any patients with more than trivial residual mitral regurgitation underwent re-exploration during a second run on bypass and this was necessary in 42 patients (5%). A third pump run was necessary in three patients (0.4%).
Perioperative characteristics and outcomes are reported in Table 2. Mean aortic cross clamp time was 95±28.5 minutes and cardio-pulmonary bypass time was 161±19.4 minutes (range, 53-505 minutes). The overall in-hospital mortality rate was 0.2% (n=2). One patient died of multi-organ failure, and the second one had rupture of the mitral annulus after mitral valve replacement was performed following failed repair in a patient with a calcified annulus. Mechanical ventilation was less than 24 hours in 807 (95.8%) patients, and more than 24 hours in 35 patients (4.2%).
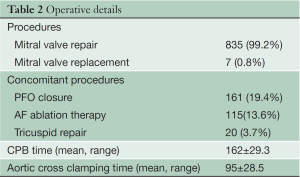
Full table
Eight patients (0.9%) suffered from stroke, while four patients (0.46%) suffered from transient ischemic attack. When stroke was subdivided into major or minor by the presence of a permanent neurological deficit, major stroke occurred in five patients (0.6%).
Perioperatively, five patients (0.6%) developed myocardial infarction due to circumflex artery obstruction, three of whom underwent emergent coronary artery bypass grafting.
After mitral valve repair, eight patients (0.9%) developed a new aortic valve insufficiency. One of these patients had an aortic valve repair through a sternotomy during the same operative session. Two patients had a delayed reoperation on the aortic valve (at two weeks and six months respectively). Five patients with a mild aortic regurgitation are regularly followed up with echocardiography.
Sixty patients (7.1%) had MAE within 30 days. The detailed causes of MAEs are presented in Table 3.
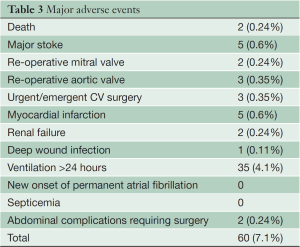
Full table
Altogether 29 patients (3.4%) had a conversion to sternotomy. In 13 patients (1.6%), conversion to sternotomy was necessary during the first operation. Causes for immediate conversion are presented in Table 4. In 16 patients (1.9%), conversion to sternotomy was necessary for a second operation. Causes for delayed conversion are presented in Table 4. Seventeen patients (2%) had a re-thoracotomy for excessive bleeding.
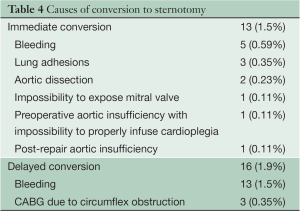
Full table
Other postoperative complications are detailed in Table 5.
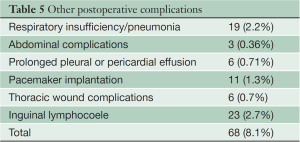
Full table
In 109 patients (12.9%), blood transfusion was given. More than two units of blood were necessary in 67 patients (7.9%), whereas 733 patients (87.1%) had no transfusion at all.
The overall mean hospital stay was 9.93±6.3 days, with 166 patients (19.7%) having a prolonged (>10 days) hospital stay.
Pre-discharge transthoracic echocardiography was performed in all 834 patients who were alive and had undergone mitral valve repair. Seven hundred and fifty five patients (90.5%) had no or trace mitral regurgitation, whereas 75 patients (9%) had mild mitral regurgitation and four patients (0.5%) had moderate mitral regurgitation.
Two patients (0.2%) had a failed repair within the first 30 days, and a reoperation was necessary. One patient had a ring dehiscence and a re-repair was possible two weeks after the first operation. The second patient with annular calcification had a valve replacement and died of a ruptured annulus.
Comments
Minimally invasive mitral valve repair for degenerative mitral valve regurgitation has long been advocated for the potential benefits that it can provide to patients.
The rate of repair of the present study was high, as in other studies (12). The rate of repair, exceeding 99%, is similar to that presented by Castillo in patients operated on conventionally through sternotomy (13). In the early part of our experience, patients were selected according to the expected difficulty of the repair. However, currently all patients with isolated mitral valve regurgitation are operated on minimally invasively, with the rare exception of patients presenting with a calcified mitral annulus. After a careful and thorough surgical valve analysis, a methodical strategy and commitment are key to obtaining a high percentage of repair. It is notable that minimally invasive surgery is not usually associated with a decreased rate of repair. As shown by Gammie et al., the rate of repair was significantly higher in the STS database, when patients were operated on with a minimally invasive approach (85% vs. 67%) (14).
Our mortality rate of 0.2% was low and similar to that in all major series of mitral valve repair (13). A high rate of repair and low operative mortality is achievable when minimally invasive surgery is performed under strict conditions: a team of professionals accustomed to working together, with no compromise to the choice of the surgical technique used. A step-by-step approach to avoid safety issues during the learning phase (15) is mandatory, as well as meticulous preparation. Our results can partially be explained by the fact that our center is a high-volume mitral valve center.
There have been concerns regarding increased risk of stroke after minimally invasive mitral surgery (14). A meta-analysis has recently shown that the risk of stroke was significantly increased for minimally invasive mitral surgery compared with standard surgery through sternotomy (2.1% vs. 1.2%) (16). No specific explanation has been provided, and the use of an endoaortic clamp was not associated with increased risk in comparison to transthoracic clamps. Femoral arterial cannulation and retrograde flow have been suspected without proof. If de-airing has been underlined as an issue, the use of CO2 and a precise technique of de-airing with echocardiographic control should bring the risk of air embolism to almost zero. A factor, which may seem minor but has never been discussed, is the way the wound of the working port is protected. Leaving adipose tissue exposed between bands of a tissue retractor increases the risk of bringing fatty debris into the heart with the continual insertion and removal of instruments. The rate of stroke (0.95%) and the risk of permanent neurological deficit (0.6%) reported in this study are low, and similar to those reported in other series for mitral valve repair performed through sternotomy.
The cross clamp time and the bypass time 95±28.5 minutes and 161±19.4 minutes, respectively. As demonstrated in other studies, cross clamp time is significantly increased with a minimally invasive approach, in comparison with sternotomy (4,5,17,18). With experience, cross clamping time has progressively decreased. It is of note that both our cross clamping time and bypass time compared favorably with the ones reported by Castillo for a group of patients treated for degenerative disease through conventional sternotomy (13). It seems that to attain a high percentage of repairs, irrespective of the approach, one has to concentrate on the mitral valve, using optimal myocardial protection, rather than focus on the burden of the time.
In this series five patients developed myocardial infarction due to occlusion of the circumflex artery secondary to the placement of the ring sutures. Practically, when operating minimally invasively, it is not possible to apply the same safe surgical methods to avoid the circumflex artery that are used during sternotomy (i.e., pulling the posterior leaflet away from the circumflex artery and suturing the annulus in the direction of the ventricle). Undisputably, a learning curve effect is present (15,19), and complications happened more frequently at the beginning of our experience. The use of intraoperative TEE as advocated by Ender (20) allows early diagnosis of complications.
Another complication, which should be a constant concern when repairing the mitral valve minimally invasively, is the occurrence of aortic insufficiency. This complication was seen in eight patients, five of whom did not require a reoperation. This complication underlines the anatomical closeness of the two valves, which should be kept in mind when placing the sutures for the ring at the level of the anterior leaflet.
It is notable that the rate of blood transfusion was low, with 87% of the patients not requiring any transfusion. This represents one advantage of this approach, considering the deleterious effects of blood transfusions (21,22).
The definition of MAEs in the present report differs somewhat from the one in the EVEREST II study (23), comparing surgery with Mitraclip®. Blood transfusion was not counted as a MAE in our study, and prolonged ventilation was considered to be more than 24 hours.
This report serves to provide data on current outcomes of mitral valve repair performed minimally invasively, but also to set a standard with which it is possible to compare emerging mitral valve repair technologies.
In conclusion, a very high rate of repair via a minimally invasive approach is achievable, with a very low rate of operative mortality, high rate of postoperative resolution of mitral regurgitation and a low rate of complications. The cosmetic results and the postoperative comfort are associated with a very high level of patient satisfaction.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III 1996;319:219-23. [PubMed]
- Dogan S, Aybek T, Risteski PS, et al. Minimally invasive port access versus conventional mitral valve surgery: prospective randomized study. Ann Thorac Surg 2005;79:492-8. [PubMed]
- Modi P, Rodriguez E, Hargrove WC 3rd, et al. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg 2009;137:1481-7. [PubMed]
- Suri RM, Schaff HV, Meyer SR, et al. Thoracoscopic versus open mitral valve repair: a propensity score analysis of early outcomes. Ann Thorac Surg 2009;88:1185-90. [PubMed]
- Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: a propensity-matched comparison. J Thorac Cardiovasc Surg 2013;145:748-56. [PubMed]
- Vollroth M, Seeburger J, Garbade J, et al. Minimally invasive mitral valve surgery is a very safe procedure with very low rates of conversion to full sternotomy. Eur J Cardiothorac Surg 2012;42:e13-5; discusson e16.
- Perier P, Deloche A, Chauvaud S, et al. Comparative evaluation of mitral valve repair and replacement with Starr, Björk, and porcine valve prostheses. Circulation 1984;70:I187-92. [PubMed]
- Mohty D, Orszulak TA, Schaff HV, et al. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation 2001;104:I1-7. [PubMed]
- Vassileva CM, Mishkel G, Mcneely C, et al. Long-term survival of patients undergoing mitral valve repair and replacement: a longitudinal analysis of Medicare fee-for-service beneficiaries. Circulation 2013;127:1870-6. [PubMed]
- Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [PubMed]
- McClure RS, Athanasopoulos LV, Mcgurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206. [PubMed]
- Castillo JG, Anyanwu AC, Fuster V, et al. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg 2012;144:308-12. [PubMed]
- Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90:1401-8, 1410.e1; discussion 1408-10.
- Murzi M, Cerillo AG, Bevilacqua S, et al. Enhancing departmental quality control in minimally invasive mitral valve surgery: a single-institution experience. Eur J Cardiothorac Surg 2012;42:500-6. [PubMed]
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [PubMed]
- Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401-5. [PubMed]
- Iribarne A, Easterwood R, Russo MJ, et al. Comparative effectiveness of minimally invasive versus traditional sternotomy mitral valve surgery in elderly patients. J Thorac Cardiovasc Surg 2012;143:S86-90. [PubMed]
- Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [PubMed]
- Ender J, Selbach M, Borger MA, et al. Echocardiographic identification of iatrogenic injury of the circumflex artery during minimally invasive mitral valve repair. Ann Thorac Surg 2010;89:1866-72. [PubMed]
- van Straten AH, Bekker MW, Soliman Hamad MA, et al. Transfusion of red blood cells: the impact on short-term and long-term survival after coronary artery bypass grafting, a ten-year follow-up. Interact Cardiovasc Thorac Surg 2010;10:37-42. [PubMed]
- Jakobsen CJ, Ryhammer PK, Tang M, et al. Transfusion of blood during cardiac surgery is associated with higher long-term mortality in low-risk patients. Eur J Cardiothorac Surg 2012;42:114-20. [PubMed]
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [PubMed]





