Techniques to define segmental anatomy during segmentectomy
Introduction
In recent years, the diagnosis of small lung nodules and non-solid lung cancers has been increasing due to developments in computed tomography (CT) technology. It is reported that the prognosis of such malignancies is good even with a sublobar resection (1-3). It is reasonable to perform a less invasive resection of a smaller volume of lung tissue, and the simple procedure of wedge resections may be sufficient if tumors are located in the peripheral sub-pleural parenchyma. However, wedge resection is inadequate for most primary lung cancers and for nodules located deep in the lung. Segmentectomy is preferred in such cases to secure an adequate surgical margin (4). In open thoracotomy surgery, a tumor is dissected bluntly by maintaining a sufficient margin while directly palpating the tumor. However, in thoracoscopic surgery, in which a hand cannot be passed directly into the thoracic cavity, it is important to proceed with the operation with a clear anatomical understanding.
Anatomical segmentectomy
In a lobectomy, demarcation of the lobar anatomy is usually relatively straightforward. In contrast, segmentectomy is more complex. In particular, the recognition of the subsegmental fissures within the pulmonary parenchyma may be difficult, with unclear boundaries between adjacent segments. In addition, when the target disease is a malignant tumor, it is necessary to secure enough surgical margin. In a thoracotomy, the tumor is dissected bluntly from the adjacent segments by maintaining a sufficient margin while directly palpating the tumor, and involved blood vessels are also treated. During thoracoscopic surgery, in which a hand cannot be passed directly into the thoracic cavity, it is important to proceed with the operation with a clear anatomical understanding.
The lung segments extend to the peripheries with the bronchus as the base. There are ten segments in the right lung (upper lobe, three; middle lobe, two; lower lobe, five) and eight segments in the left lung (upper lobe, four; lower lobe, four). Each segment has a different morphology, size and blood vessel branch, which depend on its site, and there are many variations among patients (5-7). The left upper lobe is divided into the upper and lingular divisions, while the bilateral lower lobes are generally divided into the superior and basal segment that is combined with the remaining area. As lobation is occasionally observed between these segments, the anatomy is relatively simple and easily understood. Therefore, video-assisted thoracic surgery (VATS) segmentectomy has often been performed along this plane (8,9). The problem lies with resections of other segments. It is important to plan and accurately perform the procedure (10-12). A variety of methods have been devised and used clinically, especially in thoracoscopic surgery, to solve the problem of the lack of tactile guidance (13-15).
With non-anatomical segmentectomy, the pulmonary parenchyma is roughly incised after treating the pulmonary artery and bronchus at the pulmonary hilum. However, it is not yet possible to cover resection of all segments with this method alone. The next branch of the segmental bronchus is called a subsegmental bronchus (16). Thoracoscopic resection of this subsegment has recently been performed (17). Thus, we describe herein the methods of understanding the dissection required for anatomical segmentectomy.
Understanding vascular structure
As the segmental artery is located at the pulmonary hilum in the superior segment of the lower lobe, identification and dissection are relatively easy. However, as arterial branches are embedded in the pulmonary parenchyma in some segments, it is sometimes necessary to preserve the proximal branch and divide the peripheral. Also, in many cases, more than one arterial branch is present even in a single segment. In such cases, it is useful to observe in detail and understand the morphology of the branch by employing contrast-enhanced CT, in order to carry out the surgery smoothly. A segmental artery normally accompanies the segmental bronchus. After completing division of the affected artery, the segmental bronchus can be easily traced as it is less flexible in the surrounding tissues.
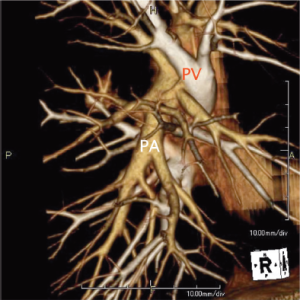
With rapid advances in multi-detector CT (MDCT) in recent years, it has become possible to easily perform three-dimensional (3D) processing not only in a workstation but also on a personal computer (Figure 1). By using MDCT, we understand each patient’s individual anatomy and can perform operations mainly by defining the course of arteries and veins (13-15). Usually, radiologists or technicians construct the 3D image using a workstation. The arteries and the veins are separately segmented and color-coded by CT value, and these volume-rendered images are then merged into the 3D-CT angiography. This image is ideal but it takes a long time to create. Thoracic surgeons know the basic anatomy of the lung, and therefore don’t need complex images. When we use volume rendering methods, we prepare simple images that meet our needs in as little time as approximately seven minutes (http://www.youtube.com/watch?v=tSO58k9Lja8). By cutting out the area of interest, the image can be magnified, de-magnified or rotated during surgery (Figure 2). We previously reported that port-access thoracoscopic segmentectomy could be safely be performed in all segments using this approach, termed Segmentectomy Achieved by MDCT for Use in Respective Anatomical Interpretation (SAMURAI) (15). Since 2004, we have performed thoracoscopic segmentectomy in 160 patients including subsegmentectomy in 20 patients, and our completion rate is 98%. The surgical results for small lung cancer are still insufficient, with a mean follow-up period of only 3.5 years as yet. However, the 5-year survival rate is 100%, which is very favorable.

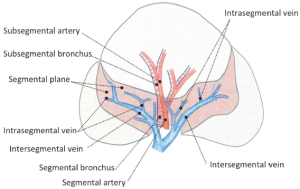
The venous branches within the segment become intersegmental veins as they converge, and return to the hilum. In segmentectomy, it is very important to understand these intersegmental and intra-segmental veins (Figure 3). The pulmonary parenchyma is dissected along the intersegmental vein, and intrasegmental vein thereby is identified. Division of the intrasegmental veins allows identification of the intersegmental border and facilitates the further parenchymal dissection (14,15). It is as if a clam can be opened when the adductor is cut.
Surgical margin
The SAMURAI method not only defines the running of blood vessels but also determines the extent of resection by virtually defining surgical margins. If it is difficult to preserve the margin in a single segment resection, we perform an extended resection of the parenchyma of adjacent segments.
Iwano and colleagues reported that radiologists propose the extent of resection to surgeons by superimposing a spherical safety margin on 3D images using a workstation for CT (18). While this method is ideal, preparing the images can be complex and time-consuming for surgeons. Although the SAMURAI method cannot create a perfect sphere in images, the surgeons themselves can evaluate resection margins intraoperatively using an appropriate scale in real time (15).
Identification of the intersegmental border
Inflation-deflation line
The basis of segmentectomy is to isolate and divide the bronchus and then dissect its peripheral pulmonary parenchyma. For conventional segmentectomy in open thoracotomy, division at the intersegmental border was generally performed by dissecting the bronchus in the affected lung and collapsing the lung on the peripheral side. In lung cancer patients, the actual method involved securing a margin by directly palpating the tumor. Meanwhile, Tsubota reported a method of inflating the affected segment to be beneficial (19). Moreover, Okada and colleagues visualized the intersegmental plane by selectively inflating the segment using a jet ventilator and reported this approach to be effective in securing an operative field. Expansion of the affected segment allows not only visualization of intersegmental borders but also maintains the morphology and size of the resected lung in the same state as the actual systemic physiological state, thereby achieving more accurate evaluation of resection margins (11). Therefore, it is considered to be more advantageous oncologically and is becoming a standard method in Japan.
Thus, jet ventilation is useful as an inflation method for the affected segment in thoracoscopic surgery or small thoracotomy. However, this method requires equipment and another doctor to maneuver the bronchoscope. Some institutions experienced such difficulties and various modifications have been devised. Direct inflation into the bronchus using a butterfly needle from the operative field was reported to be useful (20). However, great care is essential as this approach can reportedly cause air embolism (21).
We were not able to effectively insert the bronchoscope into the smaller bronchi during resection at the subsegmental (third order) bronchial branches (16). Therefore, we attempted to block the bronchus by ligation with expansion of the affected segment, especially in segmentectomy of smaller bronchial calibers. We ligated a bronchus conventionally using a knot pusher after ventilation when the bronchus was narrow. However, this method cannot be performed quickly after inflation; therefore, the affected segment will be partly deflated. We found that the monofilament slip-knot, customized from the previously reported modified Roeder knot, was useful since it enabled the surgeon to ligate the bronchus during ventilation of the lung. The bronchus is closed by pulling the thread (http://www.youtube.com/watch?v=XH2jt7kL3mo), and was effective for creating the inflation—deflation line (Figure 4) (22). We believe that this method can be generalized because it doesn’t need any special equipment and is applicable at any time.
Intersegmental veins
As described earlier, intersegmental pulmonary veins serve as important landmarks (15). The dissection of their branches, the intrasegmental pulmonary veins, facilitates intersegmental dissection. When it is difficult to reach the segmental artery and bronchus located in the deep areas of the pulmonary parenchyma, we can reach the target bronchus by dissecting the parenchyma along the intersegmental pulmonary vein. For example, in segmentectomy of S9+10 or S10 of the lower lobe, the bronchus is located in a very deep area far from the interlobar area. We have devised a posterior approach to dissecting the pulmonary parenchyma along the vein (V6) between the superior and the basal segment, initially, thereby reaching the bronchus posteriorly (http://www.youtube.com/watch?v=V2Rq92JB6vk) (23). Once the bronchus is reached, a line between the inflated and deflated areas is created using the aforementioned method. This facilitates dissection of S9 and S10, formerly classified as the most difficult segments, and reduces the operative time. As such, visualization of the line between the inflated and deflated areas and the intersegmental vein dissection are both important in performing intersegmental dissection.
Other techniques
There is a report describing a fluorescence method, wherein indocyanine green is injected into a blood vessel after treating the target segmental artery (24,25). It is based on the premise that the segmental bronchus is accompanied by the pulmonary artery. As the running vessels do not match in some cases, it is necessary to read CT images in detail to identify the pulmonary artery to ultimately be treated. A method of injecting dyes into the bronchus has also been reported (26). While this direct method is promising, it requires an additional procedure of injecting materials via bronchoscopy. Although both methods require special instruments and procedures, we anticipate that there will be further reports describing their general use in the future.
Future simulation: virtual to real
Computer technology is rapidly advancing. We are now able to visualize the surfaces of pulmonary blood vessels, output the dendritic structure as an STL file, and create a 3-dimensional solid model using a 3D printer. After sterilization, this device can be hand held and observed during surgery (Figure 5). As 3D printer equipment and consumable supplies are expensive, there is an issue of cost in creating the model. While it still cannot be regarded as an item for actual use as compared with virtual technology, there is potential for this approach to become a useful tool if the manufacturing cost can be brought down in the future.

Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [PubMed]
- Nakayama H, Yamada K, Saito H, et al. Sublobar resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg 2007;84:1675-9. [PubMed]
- Asamura H. Minimally invasive approach to early, peripheral adenocarcinoma with ground-glass opacity appearance. Ann Thorac Surg 2008;85:S701-4. [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [PubMed]
- Boyden EA. A critique of the international nomenclature on bronchopulmonary segments. Dis Chest 1953;23:266-9. [PubMed]
- Yamashita H. eds. Roentgenologic Anatomy of the Lung. New York: Igaku-Shoin, 1978.
- Ramsay BH. The anatomic guide to the intersegmental plane. Surgery 1949;25:533-8. [PubMed]
- Houck WV, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery upper lobe trisegmentectomy for early-stage left apical lung cancer. Ann Thorac Surg 2004;78:1858-60. [PubMed]
- Shiraishi T, Shirakusa T, Iwasaki A, et al. Video-assisted thoracoscopic surgery (VATS) segmentectomy for small peripheral lung cancer tumors: intermediate results. Surg Endosc 2004;18:1657-62. [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest 2005;128:2696-701. [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [PubMed]
- Nomori H, Mori T, Ikeda K, et al. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg 2012;144:87-93. [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Total thoracoscopic pulmonary segmentectomy. Eur J Cardiothorac Surg 2009;36:374-7; discussion 377. [PubMed]
- Oizumi H, Endoh M, Takeda S, et al. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg 2010;90:1382-3. [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [PubMed]
- Oho K, Amemiya R. eds. Practical fiberoptic bronchoscopy. Tokyo: Igaku-Shoin, 1980.
- Kato H, Oizumi H, Inoue T, et al. Port-access thoracoscopic anatomical lung subsegmentectomy. Interact Cardiovasc Thorac Surg 2013;16:824-9. [PubMed]
- Iwano S, Yokoi K, Taniguchi T, et al. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: technique and initial experience. Lung Cancer 2013;81:410-5. [PubMed]
- Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today 2000;30:963-4. [PubMed]
- Kamiyoshihara M, Kakegawa S, Ibe T, et al. Butterfly-needle video-assisted thoracoscopic segmentectomy: a retrospective review and technique in detail. Innovations (Phila) 2009;4:326-30. [PubMed]
- Otsuka T, Nakamura Y, Harada A, et al. Extremely rare but potential complication of diffuse brain edema due to air embolism during lung segmentectomy with selected segmental inflation technique by syringe needle during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2011;142:e151-2. [PubMed]
- Oizumi H, Kato H, Sadahiro M, et al. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg. [PubMed]
- Oizumi H, Kato H, Fukaya F, et al. A posterior approach for lateral posterior basal bisegmentectomy of the lower lobes. The Journal of the Japanese Association for Chest Surgery 2011;25:235-7.
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [PubMed]
- Oh S, Suzuki K, Miyasaka Y, et al. New technique for lung segmentectomy using indocyanine green injection. Ann Thorac Surg 2013;95:2188-90. [PubMed]