Treatment of stage I lung cancer in high-risk and inoperable patients: SBRT vs. RFA vs. sublobar resection
Background
Although surgical resection for early stage lung cancer is the mainstay of treatment, many patients are inoperable at the time of presentation due to either disseminated disease or medical comorbidities (1). Novel strategies are currently being developed to treat early-stage non-small cell lung cancer (NSCLC) in this expanding population of high-risk and inoperable patients. Stereotactic body radiotherapy (SBRT) modifies traditional radiation techniques to provide a high-dose per fraction of radiation to the tumor which is administered over a few fractions. This allows for effective tumor ablation with preservation of the surrounding tissue due to steep dose gradients. Radiofrequency ablation (RFA) utilizes CT-guided placement of a radiofrequency-emitting probe. As frictional heat energy from the probe is transferred to the tumor, cancer cells undergo coagulation necrosis.
In an effort to expand the population of operable patients, many groups are currently exploring the use of sublobar resection to treat early stage tumors. Early evidence suggests that sublobar resection may provide satisfactory oncologic outcomes while avoiding the morbidity of standard lobectomy in patients with poor pulmonary reserve (2). Three major clinical trials have been developed to investigate the use of these different modalities to treat early stage lung cancer in inoperable or high-risk patients. A recently published trial, RTOG 0236, is a North American phase II trial of SBRT in patients with stage I NSCLC deemed inoperable by a surgeon or a pulmonologist. The study showed a local control rate of 90.6% at three years, and disease-free survival and overall survival at three years were 48.3% and 55.8%, respectively (3). ACOSOG Z4032 is a phase III randomized controlled trial that compared sublobar resection to sublobar resection with brachytherapy for the treatment of stage I NSCLC. Thirty- and 90-day outcomes from this study have recently been published (4). In addition, three-year results were presented at the 2013 American Society of Clinical Oncology (ASCO) meeting, showing a similar rate of local recurrence for those treated with sublobar resection (12.8%) versus sublobar resection with brachytherapy (12.5%) (5). Overall survival was comparable between the groups (sublobar resection =71%, sublobar resection with brachytherapy =72%). Lastly, ACOSOG Z4033 is a phase II prospective nonrandomized study examining high-risk patients with stage I NSCLC treated with RFA. This study has completed accrual, but survival and recurrence data have not yet matured. We conducted a comparison of selection criteria and short-term outcomes for these three studies.
Patients and setting
Patients
This study focuses on patients with stage I lung cancer that are high risk for surgical intervention due to medical co-morbidities.
Intervention(s)
We explore the selection criteria and short-term outcomes in high risk patients treated with three different treatment modalities: SBRT, sublobar resection, and RFA.
Objective(s)
We sought to compile data from three major North American trials in order to compare the selection of patients for these three treatment options, and to provide some insight into the short-term morbidity and mortality associated with each.
Methodology
The study was a retrospective secondary analysis of prospectively collected data from three multicenter trials (RTOG trial 0236, ACOSOG trial Z4032, and ACOSOG Z4033). The data were formally requested from the RTOG and ACOSOG, and the analysis was approved by both organizations. We compared entry criteria and short-term outcomes using raw data from all three trials. Categorical data were compared using chi-square test and continuous data using the Kruskal-Wallis test. We then performed a propensity-matched analysis of patients treated with SBRT and sublobar resection (RTOG 0236 and ACOSOG Z4032). Variables including age, Eastern Cooperative Oncology Group (ECOG) performance status, percentage of predicted forced expiratory volume in one second (FEV1%), and percentage of predicted carbon monoxide diffusing capacity of the lung (DLCO%) were used to build a propensity score for patients with clinical stage IA NSCLC. These scores were developed to estimate the adjusted risks of short-term outcomes associated with the choice of treatment (SBRT or surgery).
Primary outcomes
Main results
There were 55 patients available for analysis from RTOG 0236 (SBRT), 211 from ACOSOG Z4032 (sublobar resection), and 51 from ACOSOG Z4033 (RFA). RFA patients were older than those undergoing sublobar resection or SBRT (mean age in years =75.6, 70.2, 72.5 respectively, P=0.02) (Table 1). Despite having been identified as medically inoperable according to study criteria, SBRT patients had superior DLCO% (61.6%) compared with sublobar resection (46.4%) and RFA (43.7%) (P=0.001). All patients had either T1 or T2 tumors. Twenty percent of patients treated with SBRT had T2 disease (n=11), compared with 1.4% of those treated with sublobar resection (n=3). All patients treated with RFA had T1 tumors. SBRT patients received an average of 60 Gy of radiation. In patients undergoing surgical resection for clinical stage IA disease, 29.3% ultimately had a higher stage on final pathology (pIB in 25%, pIIA in 0.5%, pIIB in 1.6%, pIIIA in 1.1%, pIIIB in 0.5%, and IV in 1.1%).
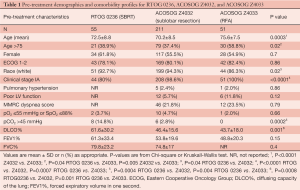
Full table
Thirty- and 90-day outcomes are shown in Table 1. For RFA, only mortality data were available. There was no significant difference in 30-day, 90-day, or treatment-related mortality amongst the three modalities. There was, however, a higher incidence of grade 3+ events at 30 days in patients undergoing sublobar resection (28.0%) compared with SBRT (9.1%) (P=0.004). The incidence was equivalent at 90 days (33.2% for sublobar resection, and 21.8% for SBRT, P=0.24). A propensity-matched score was then used to compare SBRT (n=44) and sublobar resection (n=208) in patients with T1 lesions. In the propensity-matched analysis, there was no difference in 30- or 90-day grade 3+ adverse events between these two modalities. An additional analysis was performed examining pre- and post-treatment DLCO% and FEV1% in patients treated with SBRT and sublobar resection. After adjusting for pre-treatment values, there was no difference in DLCO%. However, post-treatment FEV1% was 6.4% greater in patients undergoing sublobar resection compared with those treated with SBRT.
Study limitations
Although each of the trials was designed to evaluate patients with early stage lung cancer, subtle underlying differences in the patient populations exist. Similarly, as long-term data has not yet matured, we cannot comment on the oncologic efficacy of the treatments. In addition, our propensity matched comparison may be underpowered to detect differences in morbidity and mortality. The current analysis was meant to provide preliminary insight and definite conclusions will best be made using specifically designed, randomized controlled data comparing the modalities directly.
Applicability to other populations
These trials were designed to evaluate treatment of early stage lung cancer in high-risk or non-operable patients. The data are not necessarily applicable to patients with more advanced disease or to those who are satisfactory operative candidates.
Conclusions
Variability in patient populations in these three studies underscores the need for more reliable, objective criteria to identify the inoperable patient, the high risk but potentially operable patient, and the very high risk patient that may have a relatively better risk/benefit ratio from non-operative therapy vs. operative therapy. Our propensity-matched analysis of high-risk or inoperable patients with clinical stage I lung cancer shows no difference in 30- or 90-day mortality and morbidity between SBRT and sublobar resection. These results emphasize the need for specifically designed randomized trials to compare these treatment modalities and further stratify patients considered high risk.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45. [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg 2011;142:1143-51. [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence after sublobar resection: Results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 7502.





