Optimization of distal landing zone for TEVAR in chronic dissection
Clinical vignette
A 57-year-old male presented four years earlier with bilateral lower extremity pain with signs of malperfusion and hypertension. He was treated with emergency axillo-femoral bypass followed by aortobifemoral reconstruction. He was then treated with anti-impulse therapy and followed with regularly scheduled imaging. Comorbidities include severe obstructive pulmonary disease, atrial fibrillation, and early stage prostate cancer. He was referred for evaluation of aneurysmal degeneration in the setting of this chronic dissection. Computed tomography (CT) demonstrated a distal aortic dissection with the proximal entry tear in the descending aorta just beyond the left subclavian artery. The dissection extends retrogradely into the arch to the level of the innominate artery and distally into the iliofemoral arteries. His ascending aorta was dilated to approximately 51 mm and the proximal descending aorta expanded to 60 mm but tapered down to a normal caliber at the level of the diaphragm.
Surgical techniques
Patient selection
For patients with chronic dissection and inadequate proximal and distal landing zones, we frequently recommend a two-stage elephant trunk (ET) repair strategy including open aortoplasty and fenestration of the distal descending aorta through a retrocardiac approach at the time of the first stage and use of a stentgraft to complete the second stage (1). These patients are usually survivors of a DeBakey type I dissection after ascending repair or those with type III dissection and a dilated ascending aorta or proximal extension of the dissection into the aortic arch. For patients with more extensive disease, a staged approach is preferred to a single stage approach in order to limit the risk of spinal cord injury. Furthermore, with an endovascular second stage the delay between stages can be significantly reduced.
Preparation
Careful review of contrast enhanced three-dimensional CT imaging is required by the operating surgeon to understand the details of the patient’s anatomy and the morphology of the dissection. Before the first stage, all patients undergo preoperative cardiac catheterization for the possibility of concomitant coronary revascularization, and echocardiography to assess valve function. Additionally, patients undergo pulmonary function testing, brain imaging, and routine lab work. Between stages, little additional testing is performed except a new CT scan with contrast delivered in the arterial phase for proper sizing of the stentgraft.
Exposition
First stage repair is performed through a median sternotomy. This is often a redo sternotomy because many patients have residual dissection after prior emergency ascending repair, but this same technique has also been utilized in patients without prior proximal repair presenting with type B dissection and an inadequate proximal landing zone. Right axillary cannulation is used for arterial inflow during cardiopulmonary bypass and facilitates the use of selective antegrade brain perfusion (SABP). In the patient shown in Video 1, the axillary artery was not used because of the presence of the previous axillo-femoral bypasses. Instead, the head vessels were directly cannulated for antegrade brain perfusion. Venous cannulation is usually with a multi-stage cannula in the right atrium with or without an additional cannula in the superior vena cava to augment drainage of the head in select cases.
The patient is cooled to approximately 20 °C with bladder, esophageal, and blood temperature monitoring before commencement of SABP. The head is packed in ice, Bispectral index (BIS) is used to confirm adequate cooling and cerebral oximetry is used to confirm adequate bilateral flow. A mobile self-retaining retractor attached to the sternal retractor (Hercules; Estech, West Chester, OH, USA) retracts the innominate vein upward and away from the supra-aortic branch vessels.
A larger self-retaining retractor system is fixed to the bedrail at the level of the patient’s left hip (Omni-Tract; Integra, Plainsboro, NJ, USA). The retractor arms (“renal” pieces) are then positioned deep within the caudal portion of the mediastinum at the level of the aortic hiatus to retract the diaphragm caudally and optimize exposure of the distal descending aorta. Use of the self-retaining retraction system allows for exposure of the most distal portion of the descending aorta. In some patients, the midline incision has been extended into an upper laparotomy and the supraceliac aorta can be exposed in the lesser peritoneal sac. The use of this retractor system is paramount to achieving adequate exposure to the distal descending aorta, which can be quite difficult to see through an anterior approach.
Operation
After safe sternal entry and pericardiectomy or dissection of adhesions, the patient is cannulated as described above. After safe cannulation, cardiopulmonary bypass is initiated and cooling is begun. During the cooling phase, the additional dissection of the arch and exposure of the distal aorta as described above is undertaken. Once the patient achieves an adequate level of cooling (at least 20 minutes with electroencephalographic silence as assessed by BIS monitor), SABP is begun and the proximal aorta opened. The arch branch vessels are reconstructed first, usually with separate grafts to each vessel. The aortic arch is transected either beyond the left subclavian or at the level between the origins of the left common carotid and left subclavian arteries. The ET anastomosis is performed in the aortic arch as previously described. The largest graft possible is chosen for the ET to minimize the size discrepancy between proximal and distal landing zones for the second stage endovascular elephant trunk completion (ETC). To facilitate placement of the ET into the dissected descending aorta, it is often fenestrated from above with the ET positioned into the common channel.
During the SABP phase, attention is then turned to the previously exposed distal descending aorta. This may be approached either through an incision in the posterior pericardium or directly through the left pleural space. Using a #11 blade scalpel on a long handle, the anterior wall of the descending aorta is opened longitudinally. Long handled Pott’s scissors are then used to extend the longitudinal aortotomy for a length of about 6-8cm. Retraction stitches may be placed on either side of the aortotomy and a basket sucker is placed within the lumen to help with exposure. The membrane of the dissection flap is then identified and opened with the long #11 scalpel. The incision in the dissection flap (i.e., fenestration) is then extended as proximally and distally as seems safe to do with the Pott’s scissors. Sometimes a segment of the flap is resected, but this is not always necessary (Figure 1).
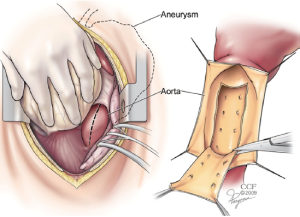
Once the fenestration has been completed, the aortotomy is closed. This is done with a two layer running 4-0 polypropylene suture supported with a strip of pericardium on either side of the incision. At least two extra-large hemoclips are placed at the top and bottom ends of the aortotomy to provide fluoroscopic markers of the distal landing zone.
Attention is then returned to the aortic arch reconstruction. The ET graft is reduced back into the mediastinum and the head vessel grafts anastomosed to the arch graft to complete the arch reconstruction. The head is de-aired. The graft is clamped more proximally, and full-flow reperfusion with warming is resumed. All suture lines, including the distal descending aortoplasty, are inspected for hemostasis prior to completing the proximal portion of the repair and any additional cardiac components of the operation.
Completion
The thoracic aortic completion repair is performed endovascularly at a later setting (0 days to 3 months, typically, depending on the anatomy and initial outcome). The benefits of staging in the patients who require a significant operation and extensive coverage of the aorta includes a lesser insult to the body at any one time and lower risk of spinal cord injury.
The day before, or morning of, the endovascular ETC, the patient receives a spinal drain. The stentgraft devices are sized based on both the diameter of the ET graft and the now modified distal landing zone. If there is a significant discrepancy in diameter then multiple components may be used to build up the system.
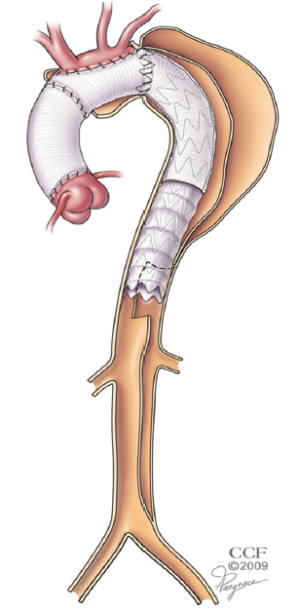
The stentgrafts are delivered from a transfemoral approach through an open cut-down. The wire access is usually achieved with a through and through technique performed with the use of a snare. A 480 cm wire is delivered from the right brachial puncture, through the ET from above and then snared from below. With the ability to place dynamic tension on the wire, the stentgraft can easily navigate the tortuosity of the ET. Very little contrast is needed for this portion of the operation because the fluoroscopic markers on the end of the ET graft and at either end of the distal fenestration provide excellent visual targets. The distal end of the stentgraft is placed at the very bottom of the fenestration, delivering balanced flow into both lumens of the residually dissected aorta without allowing any retrograde filling of the more proximal thoracic aorta (Figure 2).
Comments
Surgical intervention for distal aortic dissection has been reserved for patients who develop complications, and increasingly this has been in the form of thoracic endovascular aortic repair (TEVAR). Results of TEVAR for acute complicated dissection have been excellent as compared to open surgical or medical therapy, but the results after TEVAR for chronic complicated distal dissection are mixed (2,3). This is especially true for patients with dissections that extend into the abdominal aorta (the most common presentation).
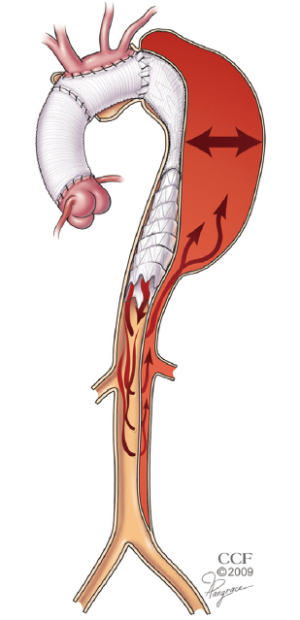
Many patients with chronic dissection have extensive aneurysm with a pattern of disease in which neither of the landing zones are ideal. Open arch repair with ET has been shown to provide a stable proximal landing zone for the placement of a stentgraft (4). When the distal landing zone has residual dissection, however, the ability to predict thromboexclusion of the treated segment is unreliable because of persistent retrograde filling and pressurization of the false lumen (Figure 3).
A novel hybrid technique involving arch and ET procedure with open fenestration of the distal landing zone in the first stage, followed by TEVAR extending from the ET to the modified fenestrated segment has been developed at our institution to treat extensive chronic dissection with aneurysm (1). An example of this operation is presented in the accompanying video (Video 1).
Clinical results
In the initial report describing our use of this technique, twenty-four patients had completed the two-stage repair. Technical success was achieved in all patients during each operation with no intraoperative deaths and no type I or III endoleaks. There was only one early death in an obese patient who had a massive pulmonary embolus on postoperative day 19, and one late death due to pneumonia 18 months postoperatively. Clinical and CT imaging assessment was performed prior to discharge, within the first 6, 12 months postoperatively, and annually thereafter. There were 11 total endoleaks: 2 distal type I, both of which underwent successful endovascular repair; 9 type II, 1 resolved with intervention, 4 without, and 4 persist with no aortic growth and are being followed expectantly. There has been no type III endoleaks.
Of the 23 survivors with imaging follow-up, 16 have demonstrated shrinkage of the aneurysm sac, 5 have been stable in size, and 2 grew. Both patients with growth had late distal type I endoleaks that were treated, and none have ruptured.
Advantages
This hybrid approach not only allows for complete thoracic aortic repair within a relatively short time frame, but it also reliably excludes flow and pressurization of the false lumen in the treated segment of aorta. By eliminating the need for an anterior or posterior thoracotomy, it probably reduces overall morbidity.
Caveats
Choosing the proper level of distal fenestration is critical. Ideally, the segment of the distal aortic landing zone should measure 4cm or less in maximum diameter based on centerline of flow imaging analysis. Use of the self-retaining retractor is very important to achieve adequate exposure to the distal descending aorta from a rather deep anterior approach. Also, this portion of the aorta is only opened during the SABP portion of the case and only anteriorly, in order to avoid the bleeding risks associated with a circumferential dissection of that portion of the aorta.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Roselli EE, Sepulveda E, Pujara AC, et al. Distal landing zone open fenestration facilitates endovascular elephant trunk completion and false lumen thrombosis for extensive chronic dissection. Ann Thorac Surg 2011;92:2078-84. [PubMed]
- Mani K, Clough RE, Lyons OT, et al. Predictors of outcome after endovascular repair for chronic type B dissection. Eur J Vasc Endovasc Surg 2012;43:386-91. [PubMed]
- Kang WC, Greenberg RK, Mastracci TM, et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg 2011;142:1074-83. [PubMed]
- Roselli EE, Subramanian S, Sun Z, et al. Endovascular versus open elephant trunk completion for extensive aortic disease. J Thorac Cardiovasc Surg 2013;146:1408-16; discussion 1416-7. [PubMed]





