Lower limb malperfusion in type B aortic dissection: a systematic review
Introduction
Type B aortic dissection (TBAD) is a life-threatening disease and its management remains challenging. TBAD is classified as acute (≤14 days) or chronic (>14 days) (1) according to the time taken for onset of symptoms. The development of complications, such as rupture, aneurysmatic dilatation or malperfusion syndrome, necessitates surgical or endovascular treatment.
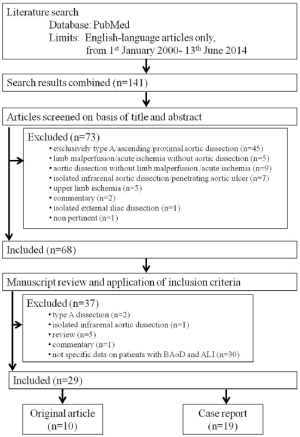
Malperfusion complications may involve renal, visceral, spinal cord and lower limb arterial circulations, inducing occlusion or thrombosis in branch arteries at high risk of end-organ ischemia. Lower limb malperfusion (LLM) is defined as abnormal pulse upon examination in conjunction with leg pain, pallor, paresthesia or paralysis (2). Recent case series (2-11) (Table 1) have reported development of LLM in 5-12% of type B dissections, in up to 40% of complicated TBAD and in up to 71% of dissections with malperfusion syndrome. The presence of LLM syndrome is statistically associated with higher in-hospital mortality (12).
Full table
The primary goal of treatment is to restore perfusion of the lower limbs as soon as possible. Extra-anatomic bypass grafting (femoro-femoral, axillo-femoral or axillo-bifemoral) or surgical fenestration represent the traditional treatment approaches. Since the 1990s, less invasive treatments employing an endovascular approach have been proposed. Occlusion of proximal entry tear with stentgraft and endovascular fenestration are two attractive options. Few studies have specifically analyzed lower limb ischemic complication because it is usually not discriminated from ischemia of abdominal visceral districts.
The aim of this systematic review is to provide clinical and procedural data of patients with acute or chronic TBAD complicated by LLM or acute limb ischemia.
Methods
Search strategy
The PubMed database was systematically searched from 1st January 2000 to 13th June 2014 for English-language publications reporting on TBAD complicated by acute limb ischemia or LLM. Search terms were “acute limb ischemia and aortic dissection” or “limb malperfusion and aortic dissection”. The reference lists of retrieved articles were also scanned to further identify potentially relevant studies. The search method and results have been reported according to the PRISMA statement (13).
Study selection
Inclusion criteria for study selection were the following: (I) reporting on LLM and/or lower limb ischemia secondary to TBAD; (II) reporting on demographics of patients with LLM and/or lower limb ischemia. Studies that did not provide demographic data for patients were not included. Case reports were eligible for inclusion, while review and commentary articles were excluded. Articles reporting exclusively on type A, ascending, proximal dissection or isolated infrarenal abdominal aortic disease were also excluded.
Two reviewers (C.B.M. and E.G.) independently screened the title and abstract of records identified in the search. Full text publications were sought and retrieved for studies that the authors agreed to be potentially relevant. Disagreements about final study inclusion were resolved by consensus (i.e., discussion between reviewers).
Data extraction
Data were extracted for the following: patient demographics (age, gender), etiology of dissection disease (hypertensive, traumatic, iatrogenic), classification of TBAD (acute: ≤14 days; chronic: >14 days), symptoms and signs (malperfusion or acute limb ischemia; chest, back, abdominal or limb pain; weakness, numbness, paralysis or paresthesia of lower extremity; peripheral pulse deficit), clinical and radiological evidence of associated renal or mesenteric hypoperfusion, type of treatment (medical, surgical, endovascular), and post-operative outcomes (technical success, 30-day clinical success, morbidity, reintervention and mortality).
Treatment type was defined as the procedure performed to treat LLM and, for this reason, hybrid treatments (e.g., aortic stentgraft associated with femoro-femoral bypass graft) were classified as surgical procedures. Technical success was defined as a procedure completed without any intraoperative complication (e.g., thrombosis of bypass graft or accidental coverage of visceral vessel by stentgraft). Clinical success was defined as absence of clinical symptoms and signs of preoperative malperfusion/ischemia at discharge. Post-operative morbidity was defined as new onset of any procedure-related complication. Thirty-day outcomes refer to either the first 30 postoperative days or period of hospitalization. Data were independently extracted by two vascular surgeons (C.B.M. and E.G.) and any discrepancies were clarified through consensus.
Results
Search results
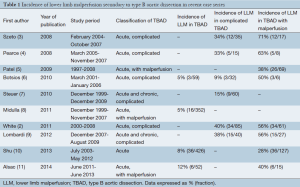
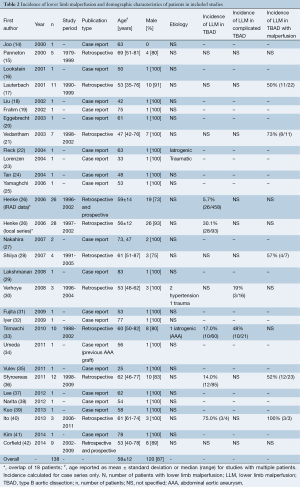
The study selection process is summarized in Figure 1. In total, 29 articles met the criteria for inclusion and their characteristics are displayed in Table 2. Of these, 10 (34%) were original articles and 19 (66%) case reports. Two original articles (26,42) only included patients with LLM syndrome secondary to TBAD. The other eight original articles (15,17,21,28,30,33,36,40) did not report exclusively on LLM syndrome, but separate data was provided for patients with LLM.
Full table
Incidence
LLM occurred in 5.7-30% of all TBAD cases, representing 19-48% of all complicated type B dissections and 50-73% of all malperfusion complications (Table 2). Among patients with previous abdominal aortic graft replacement, LLM or ischemic lower limb complications occurred in 75% of patients with TBAD (40).
Patient and etiology
There were a total of 138 patients in the included studies (with an overlap of 18 patients). This included 118 (86%) patients from original articles and 20 (14%) from case reports. The mean age of patients was 58±12 years, and 120 (87%) were male. The etiology of TBAD was rarely reported. Hypertension, trauma and iatrogenic causes were the reported etiologies in two, two and one case respectively (22,23,30). TBAD and LLM syndrome were also observed in association with untreated abdominal aortic aneurysm (29) or in patients treated with surgical graft or endovascular exclusion (32-34).
Clinical presentation and symptoms
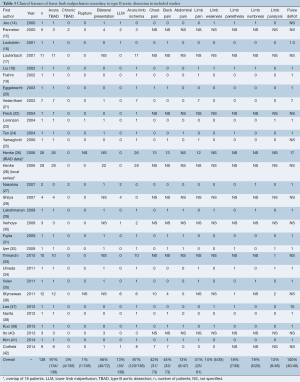
Clinical presentation and symptoms are reported in Table 3. LLM was described as a complication of acute or chronic TBAD in 134 (97%) and 4 (3%) cases, respectively. In 120 (87%) cases, LLM presented as acute limb ischemia. The other 18 (13%) patients presented with mild lower limb hypoperfusion. Bilateral clinical presentation occurred in 56% (40/72) of cases. Pulse deficit was present in all reported cases. Chest, back and abdominal pain were present in 42% (31/73), 45% (33/73) and 13% (6/47) of cases, respectively. Pain, weakness, paresthesia, numbness and paralysis occurred in 41% (25/61), 14% (5/35), 18% (7/38), 19% (5/26) and 13% (6/48) of patients, respectively.
Full table
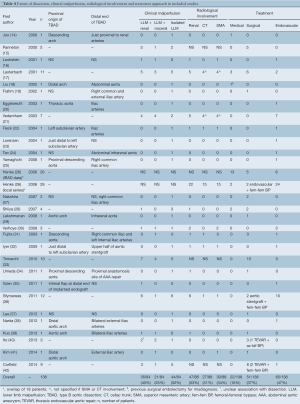
Associated malperfusions
Clinical and radiological malperfusions are reported in Table 4. In 52% (44/84) of cases, LLM was the only detected malperfusion. LLM was clinically associated with renal and visceral malperfusion in 40% (35/84) and 25% (21/84) of patients, respectively. According to radiological imaging, renal, celiac trunk and superior mesenteric artery involvement was reported in 53% (47/88), 31% (27/88) and 34% (30/88) of cases, respectively.
Full table
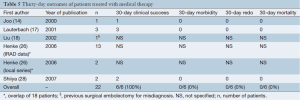
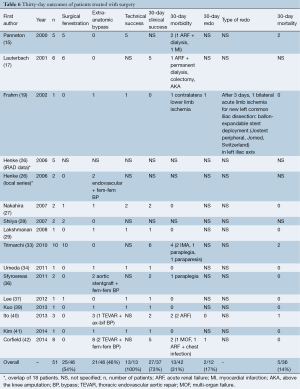
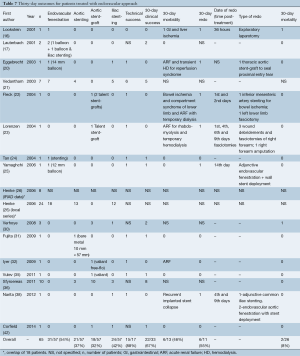
Treatment
Medical, surgical and endovascular treatments were performed in 22 (16%), 51 (37%) and 65 (47%) patients, respectively (Tables 5-7). In the surgical group, open fenestration, extra-anatomic bypass and aortic replacement were performed in 50% (23/46), 46% (21/46) and 4% (2/46) of cases, respectively. In the endovascular group, percutaneous fenestration was performed in 54% (31/57) and aortic and/or iliac stenting/stent-grafting in 95% (54/57) of cases, thoracic endovascular aortic repair (TEVAR) in 32% (18/57), aortic stenting in 37% (21/57) and iliac stenting in 42% (24/57).
Full table
Full table
Full table
Perioperative results
Medical therapy was the treatment of choice in 22 patients (16%). Thirty-day clinical success was 100% (6/6) with no 30-day morbidity and mortality (0/6).
In TBAD patients treated by a surgical approach, technical success was 100% (13/13). Thirty-day clinical failure occurred in 27% of cases (10/37) (five after surgical fenestration and five after extra-anatomic bypass). In addition, 30-day morbidity was 31% (13/42). Systemic complications included five cases of acute renal failure, three cases of myocardial infarction, two cases of paraplegia, and one case each of paraparesis, chest infection, colectomy contralateral lower limb ischemia and amputation above the knee. And amputation above the knee. Thirty-day mortality was 14% (five cases; four after fenestration and one after axillo-bifemoral bypass with associated TEVAR).
Technical success of endovascular treatment was 88% (15/17); procedure failure occurred in two cases (one case of asymptomatic thrombosis of renal artery stent and one case of endovascular fenestration with clinical worsening of the patient). Thirty-day clinical failure developed in 11 cases (33%), of which 6 (18%) were managed with TEVAR. Thirty-day morbidity was 46% (n=6), 3 (23%) of which occurred after TEVAR. Systemic complications included three cases of compartment syndrome with acute renal failure and transient hemodialysis, two cases of gastrointestinal ischemia, and one case each of liver ischemia, isolated acute renal failure and recurrent implanted stent collapse. Thirty-day mortality was 8% (2/26).
Discussion
Aortic dissection is a catastrophic event affecting the aorta and producing morphological and hemodynamic subversion in thoracic and abdominal arterial districts. TBAD represents about 38% of all aortic dissections (43). Complications occur in 20-45% of acute TBAD cases (44,45) and, in such instances, there is a dramatic increase in associated mortality (45,46). LLM occurs in 28-56% (2,47) of acute TBAD cases and, unlike renal or visceral malperfusion, is associated with poor 30-day outcomes (5).
In our systematic review, we included 29 publications reporting on a total of 138 patients with TBAD and LLM. LLM usually developed during the sixth and seventh decades of life, most frequently affected male patients, with hypertension and typically presented as acute limb ischemia (almost 90% of cases). In a minority of cases, reduced blood flow was associated with pulse deficit without leg threatening disease. In these cases, occurring generally in chronic phase, dissection causes a true lumen diameter decrease that does not result in critical reduction of arterial flow. Another possible mechanism of LLM is the movement of the intimal flap with postural changes. Nakahira et al. (27) reported two cases of atypical leg malperfusion arising due to compression of the true lumen and expansion of the false lumen upon standing.
LLM symptoms vary greatly according to the morphology of TBAD. This condition may actually present more frequently with chest or back pain than limb pain, as limb numbness, weakness or paresthesia only occurred in 15-20% of cases. Complete limb paralysis is not rare and can be caused either by severe acute limb or spinal cord ischemia.
Bilateral clinical presentation is a frequent event (56%). Flow reduction in both lower limbs can be induced by two different mechanisms: collapse of true aortic lumen (14,24,29,35) or bilateral iliac obstruction (31). Therefore, it is not only the iliac districts that should be carefully evaluated.
Isolated LLM was reported in more than 50% of patients included in the present review. However, clinical malperfusion syndrome involving other arterial districts is frequent, with associated renal malperfusion (40%) being more common than visceral (25%). Radiological involvement of the renal or visceral arteries is higher, since not all dissections lead to end-organ ischemia. Renal and visceral arteries were involved in a half and third of cases, respectively. As reported in the literature, radiological involvement of aortic branches is more common in patients with acute limb ischemia (P=0.004), and both mesenteric and renal malperfusion are significantly associated with acute limb ischemia (P=0.002 and P=0.048, respectively) (26).
TBAD and its related complications are routinely diagnosed with computed tomography angiography (CTA), while LLM can be evaluated with color duplex ultrasound. It is critical to distinguish LLM syndrome from the differential diagnosis of peripheral arterial embolism. In the case of acute onset of isolated lower limb ischemic symptoms, this distinction can be difficult. Liu et al. (18) report a case of unilateral lower limb ischemia without thoracic or abdominal symptoms or signs related with TBAD. Angiography was the diagnostic procedure performed, but iliac dissection was not detected. The patient underwent embolectomy without complete proximal progression of Fogarty catheter due to unrecognized iliac dissection. CTA finally revealed the real cause of limb ischemia.
Antihypertensive medical treatment is the first line of therapy in uncomplicated and complicated TBAD, and spontaneous recovery of LLM has been described in some cases. In the present review, 22 (16%) patients were deemed to require no interventional therapy for adequate limb perfusion. Thirty-day outcomes were only available in 6 of these cases, with optimal 30-day clinical success and freedom from mortality. As in uncomplicated TBAD, medical therapy seems to be the gold standard in the case of spontaneous resolution of lower limb symptoms. In these patients, the modification of intimal flap positioning during the acute phase could be the cause of apparent regression of the clinical syndrome.
Open surgical approach of LLM includes two different strategies: surgical fenestration and extra-anatomic bypass grafting. The former is usually performed in case of concomitant renal or mesenteric malperfusion and consists of supra-celiac or supra-renal aortic clamping and wide excision to the aortic flap in order to equalize the pressure between the true and false lumen. The latter is preferred in high-risk patients with isolated LLM. In these patients, a femoro-femoral sovra-pubic (monolateral LLM) or axillo-bifemoral (bilateral LLM) bypass graft is usually performed. Extra-anatomic bypass can be associated with endovascular deployment of aortic or iliac stent/stentgraft into the true lumen to promote false lumen collapse. In the present review, surgical fenestration and extra-anatomic bypass were reported in a similar proportion of patients. The perioperative mortality of surgical fenestration was higher than that of extra-anatomic bypass (17% vs. 5%) but morbidity was similar (30.4% vs. 31.6%). Complications may develop not only after surgical fenestration but also after extra-anatomic bypass graft, probably due to the preoperative comorbidities present in such patients.
Endovascular approach has been proposed as a less invasive procedure for TBAD since the 1990s. This approach includes TEVAR for covering the proximal entry tear with or without distal aortic/iliac stentgrafting and/or endovascular fenestration. In endovascular fenestration, septectomy of intimal septum is performed with guidewire, catheters and balloon, and sometimes with stent deployment to maintain the patency of fenestration. TEVAR with or without adjunctive distal (iliac ± abdominal aortic) stenting/endografting may induce true lumen expansion and the regression of LLM. In the present review, endovascular fenestration was performed in half of all endovascular procedures. The overall mortality of endovascular treatment was 8%; however both endovascular procedures were associated with severe complications, including gastrointestinal ischemia, acute renal failure and reperfusion syndrome.
Limitations of the study
This review has several limitations. Many studies could not be included for analysis as they omitted patient demographics. Of the included studies, several publications failed to report on outcomes and clinical and procedural data. Another key limitation was the lack of standardization in reporting data and outcomes across studies.
Conclusions
LLM syndrome secondary to TBAD was observed mainly during the acute phase (97%). In the vast majority of patients, LLM led to acute limb ischemia. Bilateral clinical presentation occurred in more than half of all cases, owing to flow limitation or thrombosis involving aorta of bilateral iliac axis. LLM was frequently associated with renal and visceral malperfusion while concomitant spinal cord ischemia was less common. Treatment of hypertension is essential and some cases of mild LLM improved with medical therapy alone. Surgical treatment displayed a higher rate of complications and surgical fenestration had higher mortality. Although the endovascular approach is less invasive, complication rates remained high.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- DeSanctis RW, Doroghazi RM, Austen WG, et al. Aortic dissection. N Engl J Med 1987;317:1060-7. [PubMed]
- White RA, Miller DC, Criado FJ, et al. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg 2011;53:1082-90. [PubMed]
- Szeto WY, McGarvey M, Pochettino A, et al. Results of a new surgical paradigm: endovascular repair for acute complicated type B aortic dissection. Ann Thorac Surg 2008;86:87-93; discussion 93-4. [PubMed]
- Pearce BJ, Passman MA, Patterson MA, et al. Early outcomes of thoracic endovascular stent-graft repair for acute complicated type B dissection using the Gore TAG endoprosthesis. Ann Vasc Surg 2008;22:742-9. [PubMed]
- Patel HJ, Williams DM, Meerkov M, et al. Long-term results of percutaneous management of malperfusion in acute type B aortic dissection: implications for thoracic aortic endovascular repair. J Thorac Cardiovasc Surg 2009;138:300-8. [PubMed]
- Botsios S, Schuermann K, Maatz W, et al. Complicated acute type B dissections: a single-center experience with endovascular treatment. Thorac Cardiovasc Surg 2010;58:280-4. [PubMed]
- Steuer J, Eriksson MO, Nyman R, et al. Early and long-term outcome after thoracic endovascular aortic repair (TEVAR) for acute complicated type B aortic dissection. Eur J Vasc Endovasc Surg 2011;41:318-23. [PubMed]
- Midulla M, Renaud A, Martinelli T, et al. Endovascular fenestration in aortic dissection with acute malperfusion syndrome: immediate and late follow-up. J Thorac Cardiovasc Surg 2011;142:66-72. [PubMed]
- Lombardi JV, Cambria RP, Nienaber CA, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg 2012;55:629-640.e2.
- Shu C, Fang K, Luo M, et al. Emergency endovascular stent-grafting for acute type B aortic dissection with symptomatic malperfusion. Int Angiol 2013;32:483-91. [PubMed]
- Alsac JM, Girault A, El Batti S, et al. Experience of the Zenith Dissection Endovascular System in the emergency setting of malperfusion in acute type B dissections. J Vasc Surg 2014;59:645-50. [PubMed]
- Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation 2003;108 Suppl 1:II312-7. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9,W64.
- Joo JB, Cummings AJ. Acute thoracoabdominal aortic dissection presenting as painless, transient paralysis of the lower extremities: a case report. J Emerg Med 2000;19:333-7. [PubMed]
- Panneton JM, Teh SH, Cherry KJ Jr, et al. Aortic fenestration for acute or chronic aortic dissection: an uncommon but effective procedure. J Vasc Surg 2000;32:711-21. [PubMed]
- Lookstein RA, Mitty H, Falk A, et al. Aortic intimal dehiscence: a complication of percutaneous balloon fenestration for aortic dissection. J Vasc Interv Radiol 2001;12:1347-50. [PubMed]
- Lauterbach SR, Cambria RP, Brewster DC, et al. Contemporary management of aortic branch compromise resulting from acute aortic dissection. J Vasc Surg 2001;33:1185-92. [PubMed]
- Liu WP, Chen WK, Ng KC. Aortic dissection presenting as acute lower extremity ischemia: report of a case. Yale J Biol Med 2002;75:211-4. [PubMed]
- Frahm C, Widmer MK, Brossmann J, et al. Bilateral leg ischemia due to descending aortic dissection: combined treatment with femoro-femoral cross-over bypass and unilateral aorto-iliac stenting. Cardiovasc Intervent Radiol 2002;25:444-6. [PubMed]
- Eggebrecht H, Baumgart D, Dirsch O, et al. Percutaneous balloon fenestration of the intimal flap for management of limb threatening ischaemia in acute aortic dissection. Heart 2003;89:973. [PubMed]
- Vedantham S, Picus D, Sanchez LA, et al. Percutaneous management of ischemic complications in patients with type-B aortic dissection. J Vasc Interv Radiol 2003;14:181-94. [PubMed]
- Fleck T, Wisser W, Cejna M, et al. Complicated acute aortic dissection type B caused by femoral cannulation for endoscopic coronary artery bypass surgery. J Endovasc Ther 2004;11:80-3. [PubMed]
- Lorenzen HP, Geist V, Hartmann F, et al. Endovascular stent-graft implantation in acute traumatic aortic dissection with contained rupture and hemorrhagic shock. Z Kardiol 2004;93:317-21. [PubMed]
- Tan ME, Morshuis WJ, Ernst SM, et al. Endovascular stenting for aortic dissection with lower extremity malperfusion. Ann Thorac Surg 2004;78:1098. [PubMed]
- Yamaguchi M, Sugimoto K, Tsuji Y, et al. Percutaneous balloon fenestration and stent placement for lower limb ischemia complicated with type B aortic dissection. Radiat Med 2006;24:233-7. [PubMed]
- Henke PK, Williams DM, Upchurch GR Jr, et al. Acute limb ischemia associated with type B aortic dissection: clinical relevance and therapy. Surgery 2006;140:532-9; discussion 539-40. [PubMed]
- Nakahira A, Ogino H, Matsuda H, et al. Postural change causing leg malperfusion resulting from expansion of a patent false lumen in type B aortic dissection. J Thorac Cardiovasc Surg 2007;134:1046-7. [PubMed]
- Shiiya N, Matsuzaki K, Kunihara T, et al. Management of vital organ malperfusion in acute aortic dissection: proposal of a mechanism-specific approach. Gen Thorac Cardiovasc Surg 2007;55:85-90. [PubMed]
- Lakshmanan R, Aung M, Hoong CK. Limb ischaemia in a Stanford B aortic dissection into an abdominal aortic aneurysm. ANZ J Surg 2008;78:660-1. [PubMed]
- Verhoye JP, Miller DC, Sze D, et al. Complicated acute type B aortic dissection: midterm results of emergency endovascular stent-grafting. J Thorac Cardiovasc Surg 2008;136:424-30. [PubMed]
- Fujita W, Daitoku K, Taniguchi S, et al. Endovascular stent placement for acute type-B aortic dissection with malperfusion--an intentional surgical delay and a possible ‘bridging therapy’. Interact Cardiovasc Thorac Surg 2009;8:266-8. [PubMed]
- Iyer V, Rigby M, Vrabec G Sr. Type B aortic dissection after endovascular abdominal aortic aneurysm repair causing endograft collapse and severe malperfusion. J Vasc Surg 2009;50:413-6. [PubMed]
- Trimarchi S, Jonker FH, Muhs BE, et al. Long-term outcomes of surgical aortic fenestration for complicated acute type B aortic dissections. J Vasc Surg 2010;52:261-6. [PubMed]
- Umeda Y, Imaizumi M, Mori Y, et al. Severe limb ischemia related to previous abdominal aortic aneurysm repair induced by acute aortic dissection. Ann Vasc Dis 2011;4:37-9. [PubMed]
- Vulev I, Klepanec A, Balázs T, et al. Endovascular treatment of late in-stent-graft dissection after thoracic endovascular aneurysm repair. Cardiovasc Intervent Radiol 2011;34:864-7. [PubMed]
- Sfyroeras GS, Rubio V, Pagan P, et al. Endovascular management of malperfusion in acute type B aortic dissections. J Endovasc Ther 2011;18:78-86. [PubMed]
- Lee CH, Chang CH, Tsai YT, et al. Isolated lower limb ischaemia as an unusual presenting symptom of aortic dissection. Cardiovasc J Afr 2012;23:e13-4. [PubMed]
- Narita K, Akutsu K, Yamamoto T, et al. Simultaneous fenestration with stent implantation for acute limb ischemia due to type B acute aortic dissection complicated with both static and dynamic obstructions. Ann Thorac Cardiovasc Surg 2012;18:158-61. [PubMed]
- Kuo HN, Lai HC, Chang YW, et al. Axillofemoral bypass relieves visceral malperfusion in type B aortic dissection. Ann Thorac Surg 2013;95:703-5. [PubMed]
- Ito T, Kawaharada N, Kurimoto Y, et al. Infradiaphragmatic malperfusion of acute aortic dissection associated with previous abdominal aortic aneurysm repair. Surg Today 2013;43:1019-24. [PubMed]
- Kim KH, Choi JB, Kuh JH. Simultaneous relief of acute visceral and limb ischemia in complicated type B aortic dissection by axillobifemoral bypass. J Thorac Cardiovasc Surg 2014;147:524-5. [PubMed]
- Corfield L, McCormack DJ, Bell R, et al. Role of the femorofemoral crossover graft in acute lower limb ischemia due to acute type B aortic dissection. Vascular 2014;22:121-6. [PubMed]
- Toda R, Moriyama Y, Masuda H, et al. Organ malperfusion in acute aortic dissection. Jpn J Thorac Cardiovasc Surg 2000;48:545-50. [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [PubMed]
- Trimarchi S, Tolenaar JL, Tsai TT, et al. Influence of clinical presentation on the outcome of acute B aortic dissection: evidences from IRAD. J Cardiovasc Surg (Torino) 2012;53:161-8. [PubMed]
- Shu C, He H, Li QM, et al. Endovascular repair of complicated acute type-B aortic dissection with stentgraft: early and mid-term results. Eur J Vasc Endovasc Surg 2011;42:448-53. [PubMed]
- Shu C, Fang K, Luo M, et al. Emergency endovascular stent-grafting for acute type B aortic dissection with symptomatic malperfusion. Int Angiol 2013;32:483-91. [PubMed]