Gastrointestinal bleed after left ventricular assist device implantation: incidence, management, and prevention
Introduction
Continuous-flow left ventricular assist devices (CF-LVADs) have become the standard of care for patients with end-stage heart failure (HF). The use of these devices has improved outcomes for patients with end-stage HF, both as destination therapy or as a bridge to cardiac transplantation (1). While these devices have improved durability compared to earlier generation left ventricular assist devices (LVADs), increased frequency in some complications has been seen, including gastrointestinal bleeding (GIB), pump thrombosis and hemolysis (2). This review focuses on the incidence, management and prevention of GIB after CF-LVAD implantation.
Background
Heart failure is an increasingly common diagnosis in the American population, with estimates as high as 500,000 new cases per year. The rise in the number of patients who develop progressive HF is thought to be due to the reduction in deaths from acute coronary syndromes, improved survival with most cardiovascular interventions and the use of implantable cardioverter defibrillators (3-5). The New York Heart Association (NYHA) classifies patients with HF by their functional status, from I (no limitation in activities) to IV (symptoms at rest). The estimated number of patients in NYHA class IV HF is 15,600 to 156,000 (6).
The mainstay therapy for years for patients with end-stage HF has been optimum medical management, but many patients develop HF that is refractory to medical therapy. If deemed to be suitable candidates, these refractory cases are placed on the waiting list for cardiac transplantation. Those who are not candidates for transplantation, or for whom an organ is not available in a timely fashion, may require mechanical support for their failing hearts with an LVAD. This device may be used as a bridge to transplantation (BTT) or as destination therapy (DT) (1).
Many studies suggest that patients with LVADs have better clinical outcomes at the time of transplant than those without such devices (7-9). Furthermore, data from the REMATCH trial indicates that LVAD treatment is a plausible alternative to medical therapy for patients who are not candidates for heart transplantation (10). Over the past decade, LVADs that use continuous-flow (CF) blood pumps have been developed in response to the need for smaller and more durable devices. Although earlier pulsatile LVAD designs provided adequate cardiac output support, their large size and limited durability, necessitating frequent re-operation, hindered long-term success. The smaller size of CF-LVADs allows extension of this life-saving therapy to underserved patient populations, including women and even some children (11). Recent studies of patients supported by CF-LVADs indicate that there are fewer device-related complications and outcomes are improved (12).
As studies continue to demonstrate the benefits and improving survival outcomes of LVAD implantation worldwide, efforts to reduce the associated complications are needed. The most common complications after implantation include stroke, right heart failure, GIB, and infection (13).
While CF-LVADs have further improved patient outcomes and decreased postoperative complications, one complication that has increased in incidence is GIB which results in significant morbidity. In this review, the incidence, etiology, management, prevention and future directions of GIB following LVAD implantation will be reviewed with a focus on our experience with nearly 300 HeartMate II (HMII) implantations at University of Minnesota over the past nine years.
Contemporary results
Incidence
Numerous studies have been published showing the incidence of GIB after LVAD implantation to vary between 18-40% (11,14-18) (Table 1). Small retrospective studies (19,20) have demonstrated significantly increased rates of GIB with the use of CF devices, such as HMII, as compared to pulsatile-flow devices. INTERMACS data have shown CF devices to have decreased rates of readmission compared to pulsatile-flow devices, but with more CF readmissions due to bleeding (9.8%) than with pulsatile devices (6.2%) (21).
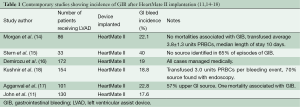
Full table
At the University of Minnesota, out of 233 patients who underwent HMII implantation between 2005-2013, 60 GIB episodes occurred in 51 patients (22%), for an event rate of 0.17 GIB/patient year of support. Patients who developed GIB were older (63 vs. 55 years, P<0.001), had lower pre-operative BMIs (26 vs. 29 kg/m2, P<0.05) and albumin levels (3.3 vs. 3.5 g/dL, P<0.5). Women made up 15% of the cohort, yet contributed 30% of the GIBs (P=0.07). There was no statistical difference in pre-operative BTT or destination therapy status, INTERMACS profile, or platelet count. There was no statistical difference in six month, one or two year survival in patients who developed a GIB and those who did not (77% vs. 78%, 74% vs. 71%, and 61% vs. 54%, respectively).
Etiology/pathophysiology
Patients with CF-LVADs receive antiplatelet medications and warfarin due to the concern for thrombosis. This was not a recommendation with the earlier pulsatile-flow devices. The observed increase in bleeding rates may be partly attributed to this need for chronic anticoagulation; however, it appears that several other mechanisms are also involved. In the case of LVADs, where the biomaterial is in direct contact with the blood circulation, significant changes in systemic immunologic and thrombostatic functions have been well documented (22).
One proposed mechanism for GIB after CF-LVAD implantation is acquired von Willebrand syndrome. Von Willebrand factor (vWF) is a protein expressed by vascular endothelial cells that plays an essential role in preventing pathological coagulopathy or bleeding. Excessive cleavage of large vWF multimers results in pathological bleeding as seen in acquired von Willebrand syndrome (23).
The association between loss of vWF multimers and GIB was first described in Heyde syndrome. This syndrome occurs in individuals with aortic stenosis who develop acquired von Willebrand syndrome and GIB from previously latent intestinal angiodysplasia. The proposed mechanism is fragmentation of high-molecular-weight multimers of vWF on the stenotic valve leading to acquired von Willebrand syndrome (24). Aortic valve replacement has been shown to improve the hematologic abnormalities in these patients (25).
A similar mechanism for acquired von Willebrand syndrome is proposed for patients with LVADs. Typical laboratory findings of an acquired von Willebrand syndrome have been demonstrated in patients with different types of CF-LVADs, including axial flow CF-LVADs such as HMII and centrigual flow CF-LVADs such as HeartWare (HVAD) (26). A recent study by Meyer et al. demonstrated the same reduction in high molecular weight multimers in centrifugal flow LVAD patients that was seen in axial flow CF-LVADs. These findings suggest that although HVAD shear forces are lower due to the contact-free design and lower revolutions per minute, they still reach a sufficient threshold to induce vWF unfolding, leading to high molecular weight multimer loss (27,28).
Another proposed mechanism for GIB after CF-LVAD implantation involves chronic low pulse pressure, which has been demonstrated in aortic stenosis to lead to AVM formation. It is proposed that intestinal hypoperfusion from reduced pulse pressure leads to regional hypoxia, vascular dilation, and subsequent angiodysplasia (19).
Along with acquired von Willebrand syndrome and the increased risk for angiodysplasia due to reduced pulse pressure, patients with CF-LVADs have also been shown to develop impaired platelet aggregation. One study showed that out of 16 patients, 11 patients with HMII had impaired ristocetin-induced platelet aggregation and a history of bleeding events. A proposed mechanism involves inhibition of platelet aggregation by vWF fragments generated from the breakdown of vWF multimers (29).
Screening of patients for angiodysplasia and von Willebrand syndrome before CF-LVAD implantation may allow for preemptive treatment and avoid potential complications postoperatively (30).
Management/diagnosis
GIB after LVAD implantation can be classified as upper GI (proximal to the ligament of Trietz) or lower GI (distal to the ligament of Trietz). Most common causes are vascular malformations like AVM and Dieulafoy lesions, which account for 30-40% and 15-20% respectively (16). Diagnosis is often made by using multiple diagnostic modalities, although the source of the bleed is not always identifiable. Such modalities include esophagogastroduodenoscopy, colonoscopy, small bowel capsule endoscopy, tagged red blood cell scan, mesenteric angiogram and deep bowel enteroscopy. Concern over electromagnetic interference from capsule endoscopy has proven unfounded (31). One recent study showed the safety and efficacy of using endoscopy for the localization and therapeutic management of bleeding in LVAD patients (32).
When managing an LVAD patient with GIB, a multi-disciplinary approach is needed. The main goals of treatment are evaluating the location, and severity of the bleed, with holding anti-coagulants and resuscitation to maintain stable hemodynamics. The average transfusion requirement for readmission with GIB is 2-4 units of packed red blood cells per bleeding patient (16). All anti-coagulants are discontinued upon admission for GIB and are restarted after complete resolution of bleeding. Some studies have reported that discontinuation of anticoagulants for prolonged periods (up to 12 months) has not led to increased incidence of thrombus formation (33). A recent study of 213 patients post HMII implantation at the University of Minnesota showed that out of 14 patients who had anticoagulation withheld (either warfarin or warfarin and aspirin) due to GIB, 13 patients did not develop any adverse thromboembolic, hemolytic or neurologic events during follow up. Only one patient developed pump thrombosis that required a device exchange after 1.3 years off warfarin (34).
Aspirin is usually restarted after cessation of the GIB. Timing of resuming warfarin depends on patient status and severity of the bleed. The goal INR post-GIB in an LVAD patient is towards the lower end of the therapeutic range (1.5-2 for HMII) (30).
Future
More research is needed on the etiology of GIB as well as the proper management of anti-coagulation pre- and post-GIB. The role of anti-coagulation, acquired von Willebrand syndrome and platelet dysfunction due to altered hemodynamics is not clearly discernable at this time.
Conclusions
While CF-LVADs have become the mainstay of treatment for patients with end-stage HF and have improved overall outcomes for these patients, one complication that has seen an increase since the change to CF devices is GIB. As shown here, GIB is a complication with considerable morbidity. Future efforts to further understand the etiology of GIB and optimize anti-coagulation are needed to improve outcomes after CF-LVAD implantation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Marcel R, Meyer DM. An overview of approved and investigational left ventricular assist devices. Proc (Bayl Univ Med Cent) 2004;17:407-10. [PubMed]
- Islam S, Cevik C, Madonna R, et al. Left ventricular assist devices and gastrointestinal bleeding: a narrative review of case reports and case series. Clin Cardiol 2013;36:190-200. [PubMed]
- Miller LW. Left ventricular assist devices are underutilized. Circulation 2011;123:1552-8; discussion 1558. [PubMed]
- Loehr LR, Rosamond WD, Chang PP, et al. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016-22. [PubMed]
- Writing group members, Lloyd-Jones D, Adams RJ, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:e46-e215. [PubMed]
- Starling RC. Advanced heart failure: transplantation, LVADs, and beyond. Cleve Clin J Med 2013;80:33-40. [PubMed]
- Aaronson KD, Eppinger MJ, Dyke DB, et al. Left ventricular assist device therapy improves utilization of donor hearts. J Am Coll Cardiol 2002;39:1247-54. [PubMed]
- Frazier OH, Macris MP, Myers TJ, et al. Improved survival after extended bridge to cardiac transplantation. Ann Thorac Surg 1994;57:1416-22; discussion 1421-2. [PubMed]
- John R, Pagani FD, Naka Y, et al. Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg 2010;140:174-81. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- John R, Kamdar F, Eckman P, et al. Lessons learned from experience with over 100 consecutive HeartMate II left ventricular assist devices. Ann Thorac Surg 2011;92:1593-9; discussion 1599-600. [PubMed]
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010;29:S1-39. [PubMed]
- Wilson SR, Givertz MM, Stewart GC, et al. Ventricular assist devices the challenges of outpatient management. J Am Coll Cardiol 2009;54:1647-59. [PubMed]
- Morgan JA, Paone G, Nemeh HW, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant 2012;31:715-8. [PubMed]
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg 2010;25:352-6. [PubMed]
- Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant 2011;30:849-53. [PubMed]
- Aggarwal A, Pant R, Kumar S, et al. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg 2012;93:1534-40. [PubMed]
- Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endosc 2012;75:973-9. [PubMed]
- Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg 2009;137:208-15. [PubMed]
- Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010;56:1207-13. [PubMed]
- Bonde P, Dew MA, Meyer D, et al. National trends in readmission (REA) rates following left ventricular assist device (LVAD) therapy. J Heart Lung Transplant 2011;30:S9.
- John R, Lee S. The biological basis of thrombosis and bleeding in patients with ventricular assist devices. J Cardiovasc Transl Res 2009;2:63-70. [PubMed]
- Suarez J, Patel CB, Felker GM, et al. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail 2011;4:779-84. [PubMed]
- Loscalzo J. From clinical observation to mechanism--Heyde’s syndrome. N Engl J Med 2013;368:579-80. [PubMed]
- Pate GE, Chandavimol M, Naiman SC, et al. Heyde’s syndrome: a review. J Heart Valve Dis 2004;13:701-12. [PubMed]
- Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail 2010;3:675-81. [PubMed]
- Meyer AL, Malehsa D, Budde U, et al. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC Heart Fail 2014;2:141-5. [PubMed]
- Crow SS, Joyce DD. Are centrifugal ventricular assist devices the answer to reducing post-implantation gastrointestinal bleeding? JACC Heart Fail 2014;2:146-7. [PubMed]
- Klovaite J, Gustafsson F, Mortensen SA, et al. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol 2009;53:2162-7. [PubMed]
- Slaughter MS. Hematologic effects of continuous flow left ventricular assist devices. J Cardiovasc Transl Res 2010;3:618-24. [PubMed]
- Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012;125:3038-47. [PubMed]
- Daas AY, Small MB, Pinkas H, et al. Safety of conventional and wireless capsule endoscopy in patients supported with nonpulsatile axial flow Heart-Mate II left ventricular assist device. Gastrointest Endosc 2008;68:379-82. [PubMed]
- Pereira NL, Chen D, Kushwaha SS, et al. Discontinuation of antithrombotic therapy for a year or more in patients with continuous-flow left ventricular assist devices. Interact Cardiovasc Thorac Surg 2010;11:503-5. [PubMed]
- Kamdar F, Eckman P, John R. Safety of discontinuation of anti-coagulation in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant 2014;33:316-8. [PubMed]





