Evaluation and treatment of pump thrombosis and hemolysis
Introduction
Not all ventricular assist devices (VADs) are fully biocompatible, thus device thrombosis has always been a significant complication. Older generation pulsatile VADs were relatively large and it was practically impossible to thrombose the entire pump and cause hemodynamic consequences. Instead, any thrombus created in the pump could be dislodged, possibly resulting in an embolic stroke. In contrast, the newer continuous flow left ventricular assist devices (CF LVADs) are much smaller and have smaller gaps between the various components of the pump. These characteristics predispose CF LVADs to thrombosis of the entire pump where the clot stays in the device, leading to increased hemolysis and device dysfunction.
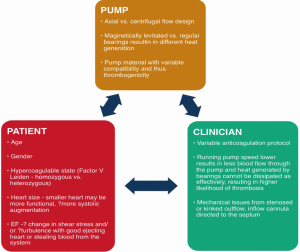
Thrombosis of CF LVADSs can occur as a result of various factors (1), which can be divided into three categories: the pump, the patient, and the clinician (Figure 1). Management protocols for VADs are usually institution-dependent, and unfortunately, there is a large variability in clinician-related factors. A decrease in anticoagulation thresholds (2-4) has been postulated to have resulted in an increase in CF LVAD thrombosis (5,6).
A complex balance exists between over-anticoagulation and under-anticoagulation, in a patient population where the coagulation system response to the CF LVAD device varies greatly between individuals. Common adverse events precipitated by over-anticoagulation include bleeding problems such as gastrointestinal bleeding and intracranial hemorrhage, while common complications due to under-anticoagulation include hemolysis, pump thrombosis and ischemic/embolic strokes. Unfortunately, robust and individually-tailored anticoagulation therapy protocols do not exist in most institutions, which usually utilize a “one size fits all” approach. In our institution, we have consistently followed an anticoagulation protocol to target the therapeutic window of coumadin of an International Normalized Ratio (INR) goal of 2-3 and full-dose aspirin for antiplatelet activity. We also follow thromboelastogram (TEG) profiles and utilize VerifyNow [International Technidyne Corporation (ITC) Edison, NJ, USA] for platelet mapping. Our protocol calls for bridging with unfractionated heparin for a goal partial thromboplastin time (PTT) of 1.5-2 times normal, which is begun on postoperative day four, provided there are no bleeding issues. In designing our anticoagulation protocol, we would rather over-anticoagulate than under-anticoagulate in the belief that bleeding complications carry a lower overall mortality and are more easily managed (7). Unfortunately, in the complex dynamics of the interaction of patients’ systems, there is no completely safe zone and we are left to “pick our poison”—thrombosis or bleeding, and sometimes both.
Clinically significant hemolysis in CF LVADs often occurs as a result of pump thrombosis, but other factors are possible:
- Dehydration or under-filled left ventricle (LV) with increased inlet velocities
- Stenotic or regurgitant native or prosthetic valves with high jet velocities
- Transfusion-associated immune and non immune-mediated hemolysis
Clinical presentation of VAD thrombosis
Patients with VAD thrombosis may present with:
- Clinically significant hemolysis. These patients usually feel unwell, and may complain of fatigue, dark urine or scleral icterus.
- Device alarms, changes in the pulsatility index, and/or increased pump power in a patient who is otherwise feeling well.
- Left heart failure. In this case, the presenting picture is dominated by pulmonary edema, hemodynamic instability, and low blood pressure. These patients require urgent evaluation and management, as they can quickly deteriorate without timely intervention.
- Fulminant cardiogenic shock in patients with totally VAD-dependent flow.
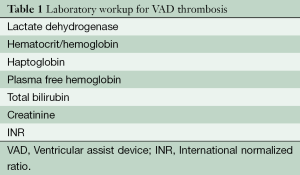
Laboratory testing (Table 1)
Full table
Lactate dehydrogenase (LDH)
The most specific indicator for device thrombosis is LDH (8), and baseline values after device implantation range from 250 to 350 IU/L. Current practice places more emphasis on the change in LDH levels rather than the actual values. Elevation of levels to more than 2.5 times that of the baseline should be investigated. If a mechanical problem leading to some degree of inflow or outflow obstruction is found, this should be surgically corrected. If no mechanical problem is found, device thrombosis should be considered and these patients should be kept well-hydrated and maintained at higher anticoagulation levels.
Hemoglobin and hematocrit (Hgb/Hct)
Hgb/Hct levels are decreased with both hemolysis and thrombosis. Significant drops from baseline may signify hemolysis; however bleeding should always be excluded.
Haptoglobin
In patients with a normally functioning CF LVAD, haptoglobin level is almost always decreased due to subclinical hemolysis. It is rather unusual if it is normal or elevated. Overall, we have not found this test helpful for the workup of VAD thrombosis.
Plasma free hemoglobin (PFH): PFH is usually elevated with pump thrombosis and hemolysis, and can be useful for workup. However, it is less sensitive than LDH levels in evaluating device thrombosis (8) with results taking several days to be delivered.
Total bilirubin (TB)
TB is usually elevated with significant hemolysis, but one needs to exclude other causes such as liver insufficiency in right heart failure, hepatitis and cirrhosis.
Creatinine
Serum creatinine can often be elevated as a result of acute kidney injury from hemoglobinuria from hemolysis and/or heart failure.
INR
Low INR levels due to inadequate anticoagulation can lead to device thrombosis. In VAD thrombosis, however, we often find INR not decreased, but rather elevated. The higher than expected INR is more an indicator of low output, and concomitant malnutrition and liver dysfunction from right heart failure, than it is a direct result of VAD thrombosis.
Diagnostic imaging (Table 2)

Full table
Chest X-ray (CXR)
Diagnostic imaging starts with a CXR. It is a readily available test that allows for the evaluation of changes in pump position, cardiomegaly and pulmonary edema.
Echocardiography transthoracic (TTE), transesophageal (TEE)
Echocardiogram is the most important diagnostic imaging modality for pump thrombosis. TTE often does not provide sufficient detail and it is probably better to obtain a TEE initially. Patients should always undergo a baseline ramp TEE after VAD implantation. It helps in determining optimal pump speed and has been shown to facilitate device malfunction detection (9). During the study, pump speed is increased until septal shift is noted, and the patient is thereafter maintained at a speed slightly below this level to allow for as much flow as possible to target LV unloading.
When patients present with suspected VAD thrombosis, a TEE should be obtained and compared to the baseline study. The relationship of the inflow cannula to the septum, lateral wall, and mitral valve should be noted. Ideally, the inflow cannula should be parallel to the long axis of the LV, as alternate angles may result in obstruction to flow. It is also critical to identify diminished or absent cannula diastolic flow velocity, or increased systolic to diastolic velocity ratio (pulsatility). Both changes have been identified as accurate predictors for suspected pump thrombosis (10). Lastly, the integrity and function of the aortic valve should be noted, as valvular insufficiency can lead to aberrant flow circuits resulting in accelerated flow velocities and turbulence, and reduced systemic flow. More important than aortic insufficiency for detecting VAD thrombosis is aortic valve opening despite ramping to a high VAD speed setting, especially if the systolic blood pressure exceeds 100 mmHg. This event signifies that the “weakened” thrombosed VAD is unable to empty the ventricle at elevated aortic pressures. If the ventricle can be emptied by increases in VAD speed, then significant thrombosis is unlikely.
Computed tomography angiography (CTA)
CTA has proven to be a useful in enhancing diagnostic evaluation of patients with suspected VAD dysfunction, and could lead to changes in patient management (11). A good quality CTA scan is able to demonstrate kinking of the outflow graft and, with three-dimensional (3-D) reconstruction, can demonstrate inflow cannula positioning. Additionally, contrast in the lumen is a sensitive marker for preserved outflow graft patency. It should be kept in mind that CTA can only diagnose mechanical problems with the outflow. It does not diagnose thrombosis, but should be considered in patients with abnormal outflow graft velocities on echocardiogram who otherwise do not appear to have pump thrombosis.
Cardiac catheterization
In addition to demonstrating intra-aortic pressures, cardiac catheterization can also demonstrate any pressure gradient in the outflow graft caused by graft kinking or stenosis. Like CTA, cardiac catheterization does not diagnose pump thrombosis and is not performed routinely, but it can be considered in patients with abnormal (increased or decreased) velocities on echocardiogram who otherwise do not appear to have pump thrombosis.
Overall, echocardiogram is the most important imaging modality in evaluating the pump. If the LV cannot be unloaded on echocardiogram, with elevated LDH, the likelihood of thrombosis is high enough to justify pump exchange. Additional imaging like CTA and cardiac catheterization should be considered if the clinical picture is unclear, and to define the area of any surgically correctable obstructions to flow.
Right heart catheterization could be considered in patients with hemolysis and heart failure symptoms, but normal or unclear unloading on echocardiogram. Right heart catheterization consistent with decreased cardiac index and elevated filling pressures despite increasing pump speed would be consistent with pump thrombosis.
Initial non-operative management
When pump thrombosis or hemolysis are suspected, anticoagulation is initiated, usually with intravenous unfractionated heparin infusion, in order to prevent clot propagation and allow for clot resolution via fibrinolysis. In addition, judicious intravenous hydration, with or without additional forced diuresis, should be used to prevent renal injury due to hemoglobinuria. Alkalization of the urine can be approached with sodium bicarbonate drips. Inotropes as indicated for heart failure symptoms are begun and the patient is medically optimized in anticipation for pump exchange. All long-acting anticoagulants are discontinued. Patients in fulminant heart failure, especially if they are VAD dependent for flow, should not be fluid overloaded; in these patients there may not be enough time for significant optimization prior to pump exchange. In patients presenting with refractory cardiogenic shock and multisystem organ dysfunction, consideration should be given to optimization with extracorporeal membrane oxygenator (ECMO) prior to pump exchange.
Identifying the problem site
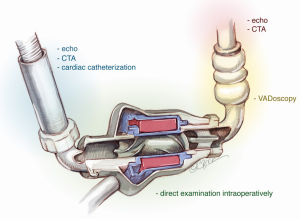
Troubleshooting the problem site in the pump involves diagnostic imaging as summarized in Figure 2.
Inflow graft
As mentioned above, echocardiography and CTA are important diagnostic modalities in determining the anatomy of the inflow cannula within the LV and its angulation towards the septum or the lateral wall. The extracardiac portion of the inflow graft, however, cannot be assessed with the imaging and can only be reliably inspected intraoperatively.
Outflow graft
CTA and cardiac catheterization have both been useful in evaluating the integrity and function of the outflow graft.
Pump or extracardiac inflow graft
Mechanical pump failure or failure of the extracardiac inflow graft are diagnoses of exclusion. This is a so-called “black box” area that cannot be examined with preoperative imaging workup. If the preoperative workup is negative the problem site is concluded to be the pump, and this can only be inspected intraoperatively.
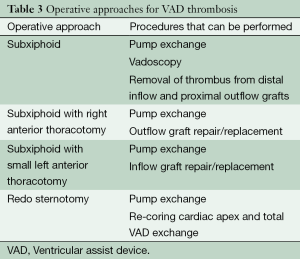
Operative approach (Table 3)
Full table
Operative VAD pump exchange can be carried out via a left upper quadrant or subxiphoid incision. The pump pocket should be reformed on every patient. Most patients require a short run of cardiopulmonary bypass. Based on the likely problem site, the specific approaches are discussed below.
Isolated subxiphoid approach
This is our primary approach for pump exchange. It allows for easy access to the pump, and parts of the inflow and outflow cannulas. In our experience, VADoscopy (12) is extremely sensitive in ruling out thrombi in the extracardiac part of the inflow cannula that are not visualized with traditional imaging modalities. If no mechanical problems or thrombus are found after careful evaluation of the inflow and outflow components, during both preoperative and intraoperative assessment, isolated pump thrombus is assumed and the pump is replaced. This is done with meticulous care to avoid dislodging the clot. During removal, control of the outflow cannula may be achieved by clamping or occluding with a Fogarty catheter.
Subxiphoid approach with right anterior thoracotomy
If the outflow graft cannot be controlled through a subxiphoid incision alone, or should the outflow graft or aortic anastomosis require revision, a right anterior thoracotomy in the third intercostal space can also be performed. This provides ample room to work on the outflow graft and perform an exchange should it be necessary.
Subxiphoid approach with small left anterior thoracotomy
If the inflow graft requires replacement and the LV apex does not require re-coring, the operation can be accomplished through a left anterior 4th intercostal incision. This approach is less morbid than a full muscle splitting subcostal incision, and works well if manipulation of the LV apex in not absolutely necessary, as the LV apex cannot be optimally mobilized and elevated through this incision.
Full redo sternotomy
If the patient goes to the operating room with a diagnosed inflow cannula positioning problem, then full sternotomy would be the preferred approach, as the apex often requires re-coring. This approach allows for full mobilization and elevation of the LV apex, with excellent exposure.
Acknowledgements
Disclosure: HTM is a consultant for Thoratec Corporation (Pleasanton, CA, USA), and SynCardia Systems, Inc. (Tucson, AZ, USA).
References
- Uriel N, Han J, Morrison KA, et al. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant 2014;33:51-9. [PubMed]
- Slaughter MS, Naka Y, John R, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Heart Lung Transplant 2010;29:616-24. [PubMed]
- Slater JP, Rose EA, Levin HR, et al. Low thromboembolic risk without anticoagulation using advanced-design left ventricular assist devices. Ann Thorac Surg 1996;62:1321-7; discussion 1328. [PubMed]
- Netuka I, Litzler PY, Berchtold-Herz M, et al. Minimal adverse events in HeartMate II patients with no antiplatelet therapy: preliminary results from the European TRACE study. J Heart Lung Transpl 2014;33:S11.
- Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant 2014;33:12-22. [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [PubMed]
- Morgan JA, Paone G, Nemeh HW, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant 2012;31:715-8. [PubMed]
- Shah P, Mehta VM, Cowger JA, et al. Lactate dehydrogenase is superior to serum-free hemoglobin as a marker of pump thrombosis in left ventricular assist devices. J Am Coll Cardiol 2013;61:E665.
- Uriel N, Morrison KA, Garan AR, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol 2012;60:1764-75. [PubMed]
- Fine NM, Topilsky Y, Oh JK, et al. Role of echocardiography in patients with intravascular hemolysis due to suspected continuous-flow LVAD thrombosis. JACC Cardiovasc Imaging 2013;6:1129-40. [PubMed]
- Acharya D, Singh S, Tallaj JA, et al. Use of gated cardiac computed tomography angiography in the assessment of left ventricular assist device dysfunction. ASAIO J 2011;57:32-7. [PubMed]
- Cheng A, Swartz MF, Massey HT. VADoscopy: a novel intraoperative technique to evaluate HeartMate II left ventricular assist device inflow obstruction and thrombosis. ASAIO J 2013;59:671-4. [PubMed]