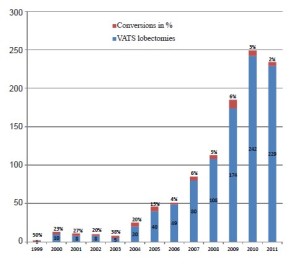
Learning curve associated with VATS lobectomy
Introduction
The first VATS lobectomy was performed in 1991 (1). Since then the implementation of VATS lobectomy has been rather slow. Data from the STS database shows a 32% rate of VATS lobectomies in 2006 (2). But it is only the best academic units that report to the STS database. This percentage is hence probably not representative for all the thoracic units in the USA. The implementation in Europe has been even slower than in the USA. But in the past years interest is rising, and in a recent report from The Society for Cardiothoracic Surgery in Great Britain and Ireland, the percentage of lobectomies performed by VATS has increased from 7% to 14 % in just one year (2010). The slow adoption despite the obvious advantages is considered by many to be due to a demanding learning curve. The procedure is considered technically demanding and has the risk of uncontrollable bleeding.
The introduction of VATS lobectomies in the surgical community was performed by self taught surgeons experienced in open surgery. The approaches varied from anterior, inferior to posterior, using 2-5 ports (3-6). These surgeons were pioneers and in case of intraoperative difficulties, conversion was their only option. The conversion rate was in many cases rather high (6). In Figure 1, the conversion rate and number of VATS lobectomies in Copenhagen between 1999 and 2011 is illustrated. The conversion rate declines with experience and number of cases per year. In the centres of the pioneers, the next generation learned the technique under guided supervision. The conditions for those surgeons’ learning curves were better due to the possibility of learning under supervision by an experienced VATS surgeon and a better possibility for selecting cases suitable for a training surgeon. Furthermore the surgical outcome was very satisfactory with low conversion and complication rates (7,8).
Since the introduction of VATS lobectomy in 1991, there has been a substantial improvement in the image quality. The introduction of firstly the digital thoracoscopes and later high definition (HD), has made precise dissection close to major vessels possible. Furthermore, several companies have designed curved instruments tailored to VATS surgery and a continuous improvement in these instruments have made it easier to perform and learn the technique. The quality of staplers has also improved significantly resulting in less air leak and fewer bronchial leaks.
The length of the learning curve
The length of the learning curve has been suggested to consist of 50 VATS lobectomies (9). But several factors influence the length of the learning curve. As mentioned previously there is a difference in the learning curve between the surgeon who takes up the procedure from scratch and the surgeon who is taught in a centre with experienced VATS surgeons to supervise. The size of the centre and the potential number of VATS lobectomies to be performed influence the length of the learning curve. Once you begin with a new technique it is an advantage to perform many operations within a short time frame. If there is only a potential to perform 1 or 2 operations a month, it will take a long time to complete a learning curve. It will be like starting all over every time. Furthermore a high case load adds the potential of selecting cases for training. The experience of the surgeon in training is important. Understanding the anatomy of the lung and experience with the many anatomical variations makes the learning curve shorter. Experience with other VATS procedures such as wedge resections, pleural biopsies and cyst resections is an advantage with respect to port placement and working in a monitor based setting.
It is an ongoing discussion about how the coming generations are going to learn to do VATS lobectomies. Once most lobectomies are performed by VATS, the lobectomies scheduled for thoracotomy may well be the difficult procedures only such as bronchial and vascular sleeves and chest wall resections. To teach such cases of open surgery to inexperienced surgeons can be demanding. One solution could be to let the inexperienced open surgeon do part of the operation. In our experience, we have had success in teaching VATS lobectomies to trainees with limited open experience given sufficient supervision and selecting the cases carefully. Finally, there are individual differences and some surgeons have more talent for the procedure and learn more quickly than others.
Simulators
The introduction of simulators of VATS lobectomy is supposed to make the learning curve of VATS lobectomies shorter. Simple simulators with an animal model, usually a porcine heart-lung tissue block filled with ketchup in a box, can simulate real surgery very well. This is a model used to train US thoracic surgery residents in VATS techniques (10). Other models used in formal VATS courses include VATS procedures on anaesthetized pigs. Although these models are effective at procedural teaching, they are limited by the cost and single use of animal tissue and the need for a thoracic surgeon to instruct. Virtual reality simulators have become an increasing popular modality for surgical education within recent years. In a recent randomised controlled trial of training with a virtual simulator developed for laparoscopy, the performance level of novices was increased to that of intermediately experienced laparoscopists and operation time was halved (11). The idea of letting residents practice with the simulator before doing surgery with improvement of cognitive and procedural skills can potentially lead to better patient safety. A recently developed virtual reality simulator uses a model for a right upper lobe lobectomy by VATS. Various anatomic variations and anomalies are randomized and loaded to present a unique surgical experience for each operation. The software is designed to identify common errors in procedural flow, including tears in pulmonary parenchyma that would result in air leaks, inappropriate ligation of vessels or bronchus to close to the pulmonary hilar origin, ligation of the vessels to the middle lobe or inferior lobe, and failure to ligate vessels to the right upper lobe. The model includes lymph node dissection (12). Virtual reality simulators will most likely play a significant role in the future training in VATS surgery. There seems to be many benefits. The amount of training is unlimited. The cost for each procedure is small, once the investment in the simulator is made. Performance scoring can be used for validation and credentialing. When introduced to surgery on patients, the surgeon is familiar with the tools and the steps of the procedure, which should enhance patient safety. We expect the learning curve in VATS lobectomy to be shorter once virtual reality simulators are introduced in the training programs.
Recommendations for a VATS lobectomy program
Before embarking on a VATS lobectomy program it is important to consider the local organisation. We believe it is important to have the potential to perform at least 25 VATS lobectomies a year. Furthermore each surgeon should have the potential to perform at least 25 VATS lobectomies a year. So given a limited number of potential VATS lobectomies, a limited number of surgeons should perform the operations. Especially in the beginning when introducing VATS lobectomy in a clinic, it is recommended to keep the VATS lobectomies on “a few hands” in order to complete learning curves for the first generation of VATS surgeons within a reasonable time. Then depending on the size of the clinic, the next generation of VATS surgeons can be trained in a supervised setting.
We have a set of recommendations for a surgeon embarking on a VATS lobectomy program (Table 1). Many different approaches to VATS lobectomy have been presented. We believe it is of utmost importance to choose one approach, and stick to it. If an anterior approach is chosen and the surgeon is used to perform a posterolateral thoracotomy, it is recommended to shift to anterior thoracotomy for a while, in order to become familiar with the hilar structures from an anterior approach. Performing more than a 100 minor VATS procedures like pleural biopsies, cyst resections and wedge resections is an advantage as the surgeon will get familiar with the port placement and working with the VATS tools in a monitor based setting. Furthermore it is highly recommended to take VATS courses and visit clinics with experience in VATS lobectomy, to observe the procedure or alternatively a fellowship in a clinic with a high volume in VATS lobectomy. Once these preparations are made, introduction can be performed in a stepwise manner. The first cases can be performed with an anterior thoracotomy, but without the use of a rib retractor. Conversion can be made very quickly and safely. Once more experience is gained, the incision can be minimized. Furthermore the procedure of the lobectomy itself can be introduced in a stepwise manner. Dividing the pulmonary ligament first, and then dissecting the vein and later turn to dissecting the arteries. Selecting the lobes is important in the beginning. Lower lobes are usually easier than upper lobes as there are fewer vessels to dissect. Small peripheral tumours are easier than central and larger tumours as the access to the hilar structures is easier. If there is suspicion of lymphatic spread, VATS lobectomies should not be attempted in the early phase of a learning curve. Previous thoracic surgery, tuberculosis or other inflammatory diseases known to give adhesions can be a difficult task for an inexperienced VATS surgeon. Incomplete fissures can be managed with a fissureless lobectomy, but there is a considerable learning curve to that technique as well. When dissecting, we recommend dissecting thoroughly in order to have a complete overview of the anatomy before dividing the vessels and bronchus, minimizing the risk of failure. Furthermore it is recommended to collect data for a prospective database to ensure quality control.
| Table 1 Recommendations for the introduction of a VATS lobectomy program |
| Perform open lobectomy by an anterior thoracotomy |
| Perform >100 minor VATS procedures |
| Attend formal courses in VATS lobectomy |
| Visit clinic with experience in VATS lobectomy |
| Training on simulators |
| Chose one approach |
| Consider volume/clinic/surgeon |
| Select patients |
| Stepwise introduction to surgical procedures |
| Prospective data collection |
We hope that in the near future every patient with early stage lung cancer, suitable for a VATS lobectomy will be offered the procedure.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7.
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54.
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6.
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5.
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402.
- Hansen HJ, Petersen RH, Christensen M. Videoassisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9.
- Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg 2010;37:516-20.
- Ferguson J, Walker W. Developing a VATS lobectomy programme--can VATS lobectomy be taught? Eur J Cardiothorac Surg 2006;29:806-9.
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80.
- Meyerson SL, LoCascio F, Balderson SS, et al. An inexpensive, reproducible tissue simulator for teaching thoracoscopic lobectomy. Ann Thorac Surg 2010;89:594-7.
- Larsen CR, Soerensen JL, Grantcharov TP, et al. Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ 2009;338:b1802.
- Solomon B, Bizekis C, Dellis SL, et al. Simulating videoassisted thoracoscopic lobectomy: a virtual reality cognitive task simulation. J Thorac Cardiovasc Surg 2011;141:249-55.