Robotic-assisted pulmonary resection - Right upper lobectomy
Introduction
General thoracic surgery is the fastest growing sector of robotic surgery. The reason is advantage the robot offers in mediastinal work such as thymectomy, resection of esophageal leiomyoma, removal of bronchogenic or esophageal duplication cysts, and even diaphragmatic plication. Once general thoracic surgeons try the robot and see the improved visualization they are often willing to continue to learn to do more with it. We have now applied it to pulmonary resections.
There are multiple published articles that have shown the efficacy and safety of robotic pulmonary resection including lobectomy, segmentectomy, and even several reports of pneumonectomy (1-4). However, there are difficulties in learning robotic surgery. It is a “team sport” where the bedside assistant is the one currently placing the stapler on the arteries and the veins, which makes everyone anxious. Another difficulty relates to the high capital cost of a robotic surgery program, including purchasing a robot, the additional expenses of buying a second console and replacing robotic surgical equipment and finally getting time on the robotic platform for the patients.
Despite the debate, cardiac and thoracic surgeons are currently learning many robotic surgery techniques. We recently helped design and develop a CPRL-4 technique and have published the world’s largest experience with it - in over 100 lobectomies. We now have completed over 180 robotic lobectomies with only one 30 or 90 day mortality. In addition, with other authors, we have written an international nomenclature paper on this issue (JTCVS 2012, publication pending) and have proctored many surgeons and trained two robotic surgery fellows. We have also published the largest series on robotic Ivor Lewis esophageal resection with a two-layered hand-sewn anastomosis. In addition, we have the world’s largest series on the robotic resection of posterior mediastinal tumors.
Based on our experience, we know all too well the difficulties in establishing robotic programs in North America. Some of these difficulties include: anesthesia push- back because of the safety concerns, and increased time, the limited degree of robot platform availability, and the fact that teams are best if they perform several robotic operations a week to get experience. In this Art of Operative Technique Teachers’ Section, we will display the specific step-by-step approach for a robotic right upper lobectomy.
Operative techniques - robotic right upper lobectomy
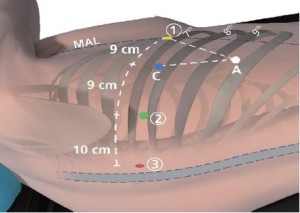
Port placement (Figure 1)
Start with the creation of a 5 mm port to facilitate port placement in the midaxillary line (MAL) placed over the 7th rib into the 6th intercostal space (ICS). Later this will become da Vinci Instrument Arm
 port.
port.Use a 5 mm videoscope through this port to ensure entry into the pleural space and to visualize placement of da Vinci and assistant ports. Start CO2 insufflation (warmed and humidified) to displace the diaphragm inferiorly.
Mark the spinous processes of the vertebral bodies on the patient (grey zone in Figure 2). Did not understand the grey zone! Perform a paravertebral block posteriorly with a local anesthetic (21 gauge needle) from ribs three to eleven under the pleural surface (0.25% Marcaine with epinephrine).
da Vinci Instrument Arm
 Port, 5 mm (Red): Place port in the ICS that is two rib spaces inferior to the major fissure and slightly anterior to the spinous process of the vertebral body. Distance to da Vinci Instrument Arm port should be at least 10 cm.
Port, 5 mm (Red): Place port in the ICS that is two rib spaces inferior to the major fissure and slightly anterior to the spinous process of the vertebral body. Distance to da Vinci Instrument Arm port should be at least 10 cm.da Vinci Instrument Arm
 Port, 8 mm (Green): Placed in the 7th ICS. Distance to da Vinci Instrument Arm
Port, 8 mm (Green): Placed in the 7th ICS. Distance to da Vinci Instrument Arm port is 10 cm and to the camera port should be at least 8-9 cm. If stapler access from this location is deemed necessary, dilate this port to a 13 mm da Vinci cannula during the surgery.
port is 10 cm and to the camera port should be at least 8-9 cm. If stapler access from this location is deemed necessary, dilate this port to a 13 mm da Vinci cannula during the surgery.Assistant Port, 15 mm (White): Use a small 21-gauge needle to identify the most anterior and inferior aspect of the chest that is just above the diaphragmatic fibers. Port location should be chosen so that a triangle is established with Camera Port and Arm
 Port with the Assistant Port at the tip equidistant to each port. It should be two or three ribs lower than and as distant to the da Vinci ports as possible to maximize assistant workspace. Keeping this port off the trajectory lines for those ports will facilitate the Patient-side assistant’s access for retraction, etc.
Port with the Assistant Port at the tip equidistant to each port. It should be two or three ribs lower than and as distant to the da Vinci ports as possible to maximize assistant workspace. Keeping this port off the trajectory lines for those ports will facilitate the Patient-side assistant’s access for retraction, etc.
Right upper lobectomy
Instrumentation: 0° and/or 30° down endoscope, 5 mm Thoracic Grasper (left
 ), Cardiere Forceps (left
), Cardiere Forceps (left  ) and Permanent Cautery Spatula or Curved Bipolar Dissector (right
) and Permanent Cautery Spatula or Curved Bipolar Dissector (right  )
)First inspect the pleural space and explore to ensure that there are no metastatic lesions on the diaphragm or the parietal or visceral pleura.
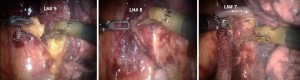
Dissection is started at the N2 mediastinal lymph nodes. If the lung deflates well the nodes #9, #8 and then #7 can be completely removed (Figure 3). If the lung does not deflate sufficiently it is best to start at the #7 station and then move cephalad toward the trachea and remove #10R and separate the azygous vein off of the trachea. Removal of the lymph nodes first opens up the anatomy and affords visual inspection of the N2 nodes.
The dissection is carried down between the hilar structures and the phrenic nerve.
Sweep phrenic nerve gently down to remove the #10R lymph node avoiding the small phrenic vein that goes to the large #10R lymph node that is routinely found in this area.
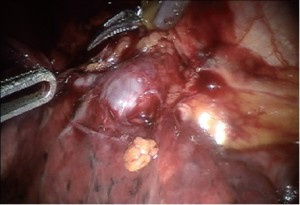
Develop the bifurcation between middle and upper lobe veins by bluntly dissecting it off of the underlying pulmonary artery. It can be easily encircled with the Cardiere Forceps or Curved Bipolar Dissector and a vessel loop; and subsequently stapled with a vascular stapler (Figure 4).
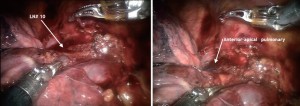
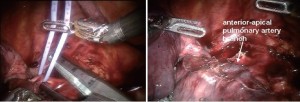
The #10R lymph node between the anterior-apical pulmonary artery branch and the superior pulmonary vein should be removed or swept up towards the lung. This exposes the anterior apical pulmonary artery branch (Figure 2).
Continue en bloc dissection of the hilar tissue to cleanly expose the main pulmonary artery.
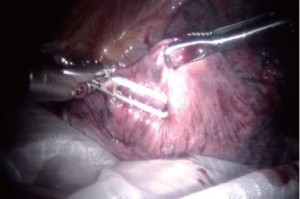
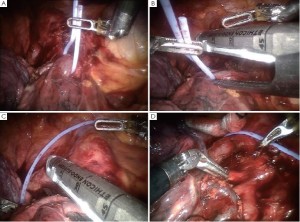
Encircle the superior pulmonary vein with an 8 cm vessel loop and retract it off the pulmonary artery behind it. Using the vessel loop as a guide, the linear stapling device is passed across the right superior pulmonary vein and fired (Figure 5 A-D).
Next the anterior apical trunk pulmonary artery branch is encircled with a vessel loop and transected with a linear stapler in the same fashion as the vein (Figure 6). Exposure might be improved by using the left hand EndoWrist instrument to deflect the trachea downward and enable the tip of the stapler device to go above the trachea.
The operation is now changed to a posterior approach in contrast to continue this anteriorly as done commonly via VATS lobectomy.
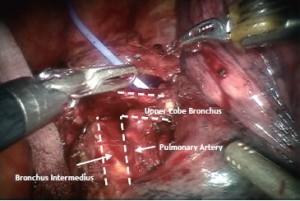
The RUL bronchus’ anatomy is exposed from posterior one. This is not possible or difficult to do with VATS in an anterior to posterior approach. However, the robot allows us to operate from either ways as seen here. The upper aspect of the RUL bronchus is easily seen coming off the trachea. The dissection is continued inferiorly to expose the inferior edge of the RUL bronchus and free it from the bronchus intermedius. Once the anatomy is identified, a Cardiere Forceps can be placed under the RUL bronchus to confirm complete dissection (Figure 7).
Lymph node dissection (10R and 11R, hilar and interlobar) is continued along the right main bronchus and the bifurcation between the bronchus intermedius and the upper lobe bronchus identified (Figure 8).
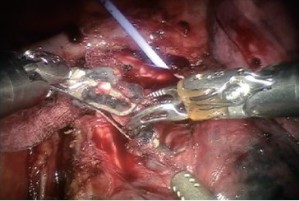
Encircle the right upper lobe bronchus with a vessel loop and transect with a linear stapler (gold or purple load). Care must be taken to apply only minimal retraction on the specimen to avoid tearing of PA branches (Figure 9).
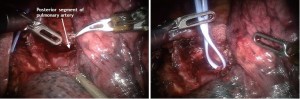
Next the posterior segment of the pulmonary artery is exposed. The surrounding N1 nodes can be removed and the posterior artery can be encircled with a vessel loop and taken with a vascular stapler. A vessel-sealing device or Titanium clips applied by the EndoWrist Small Clip Applier could be used if the vessel is less than 6 mm in size (Figure 10).
Prior to finishing the operation by stapling the fissure last, the anterior aspect of the pulmonary artery is carefully inspected to ensure that there are no PA branches remaining. If so these are usually quite small and can be easily torn and hence must be carefully ligated.
The fissure between the right upper lobe and the right middle lobe is now taken with a gold or purple stapler (Figure 11). Usually this is done anterior to posterior, however if the space between the PA and the Right Middle Lobe vein is already developed it can be done in the reverse direction as shown in Figure 11.
As the fissure is completed the main pulmonary artery should be seen and the stapler should be placed just above it and again ensuring that all small PA branches to the RUL have been taken. The right middle lobe PA branch can be easily seen and should be preserved. The RUL must be lifted up to ensure the specimen bronchus is included in the resected specimen.
To delineate the minor fissure, the upper lobe is retracted superiorly and the middle - lower lobe pushed inferiorly (Figure 12).
Minor fissure is divided with a gold or purple load linear stapler (Figure 13).
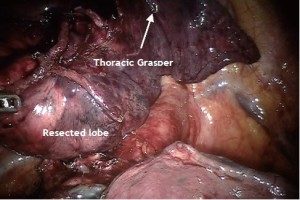
The lobe, now free of any attachments is placed remotely anteriorly and the remaining LN dissection of station 2R and 4R should be performed (Figure 14).
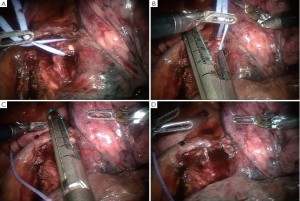
Specimen removal
Instrumentation: 0° endoscope, 5 mm Thoracic Grasper (left
 ), Cardiere Forceps (left
), Cardiere Forceps (left  ) and 5 mm Thoracic Grasper (right
) and 5 mm Thoracic Grasper (right  ) With completion of the lymph node dissection and the lobe completely resected, an “Anchor” bag is inserted into the chest from the assistant port in the 9th ICS (Figure 15).
) With completion of the lymph node dissection and the lobe completely resected, an “Anchor” bag is inserted into the chest from the assistant port in the 9th ICS (Figure 15).The lobe is then held up freely in the dome of the chest by the Thoracic Grasper. This is to utilize gravity to facilitate bagging of the lobe (Figure 16).
The open Anchor bag is placed below the freely hanging lobe (Figure 17).
The lobe is then dropped and pushed into the bag. Visualize that the complete specimen is contained in the bag while the assistant slowly closes the “Anchor” bag (Figure 18 A-C).
The straps of the bag are brought out though the 15 mm access port.
A small 20 Fr chest tube is placed apically and posteriorly via the most anterior port and guided into position by the EndoWrist instrument in arm
 . Once completed, CO2 is turned off and the right thorax vented.
. Once completed, CO2 is turned off and the right thorax vented.EndoWrist instruments are removed, the da Vinci arms are undocked and Patient cart pushed back.
Extend the assistant port in the 9th ICS to an appropriate size needed to remove the tumor en bloc.
Pull tissue straight out of thoracic cavity. Once specimen is removed use traditional VATS if needed:
Fill chest with warm saline solution, expand lung to 20 cm H2O and check for air leaks if not done already previously.
If one is found a 5-0 polypropylene with an RB-1 needle can be used to provide an airtight closure of the bronchial stump.
Chest tube is employed as per surgeon’s standard routine.
Close incisions with absorbable suture:
Skin closed with subcuticular absorbable suture without knots.

o Check for bleeding
o Check cannula sites under endoscopic view for hemorrhage.
o All cannula sites 8 mm or greater with size 0 suture at the fascia level
Comments
As shown above via the specific operative techniques, pictures, and graphics there is outstanding visibility of the anatomic structures during robotic surgery. Many people worry about encircling the vessels because of the lack of proprioception; however, the reality is that the enhanced visibility allows one to start with a blunt instrument such as a Caudier in a safe plane. The key is starting in a safe plane. For example, when encircling the superior pulmonary vein it is best to dissect the middle lobe vein from the upper lobe vein. Then identify and dissect the plane of the upper lobe vein off of the underlying pulmonary artery. The entrance point for the blunt Caudier and the exit point for the blunt Caudier should be clearly identified. Then the clamp is gently placed just under the vein and you can clearly see it come under the view and above the artery. The key to doing this safely is by first dissecting out both the entry and exit part; secondly, by using the blunt instrument (such as a Caudier; and thirdly, by having a vessel loop and rolled up Ray-Tec available to dissect the tissue off of the clamp as it comes under the vein and a Ray-Tec so compress is immediately available in case of injury and bleeding. Then a vessel loop is placed under the vessel. The vessel is retracted upwards in order to dilate the space with an open Caudier that is gently spread under the vessel. We prefer to use the vessel loop to help guide the stapler around.
The bottom line is the future of robotic surgery is extremely bright. Multiple new instruments are coming to market soon to make the operations safer and more efficient. There are even new robotic surgical techniques being developed, including the use of FIREFLY, immunofluorescence, and fluorescence of specific antigens and perhaps organs (such as the thymus). Careful studies are necessary to provide a responsible cost-benefit analysis of this interesting and exciting era of robotic thoracic surgery.
Acknowledgements
Disclosure: Robert J. Cerfolio has lectured and proctors for Intuitive (Sunnyvale, CA). Ayesha S. Bryant declares no confict of interest.
References
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9.
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6.
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25.
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95, vi-vii.