Minimally invasive and alternative approaches for long-term LVAD placement: the Vanderbilt strategy
Introduction
Left ventricular assist device (LVAD) therapy is an effective strategy to bridge patients with end-stage decompensated heart failure (HF) to orthotopic heart transplantation (OHT) (1). Advances in LVAD pump technology, evolving from bulky pulsatile devices to smaller continuous-flow (CF) pumps, combined with a better understanding of patient management, have allowed progressive improvement in long-term outcomes and pump durability after implantation (2,3). Mechanical circulatory support using the centrifugal CF HeartWare ventricular assist device (HVAD; HeartWare International, Inc., Framingham, Mass, USA) and the axial continuous-flow HeartMate-II LVAD (HM-II, Thoratec, Pleasanton, CA, USA) are the two most commonly implanted pumps in the contemporary era. Radical differences in pump design and standard implantation techniques between HVAD and HM-II LVADs have initiated the quest for pioneering surgical approaches focusing on the development of alternative and less invasive implantation techniques (4-9).
The development of minimally invasive CF-LVAD surgical techniques represents an emerging paradigm shift for the surgical and medical management of advanced HF patients. Several newer surgical approaches have been described for HVAD and HM-II CF-LVAD implantation (10,11). Due to the intrapericardial implant position and the smaller pump volume of the HVAD system, several case reports and small series of successful off-pump implants and less invasive alternative approaches have been published (5,8). For the HM-II LVAD, the relatively larger pump volume and surgical creation of a pump pocket have limited the development of minimally invasive techniques; however, several groups have reported successful attempts at thoracotomy and diaphragmatic approaches and even off-pump implantation strategies (7,9,12). We present an in-depth review and discussion of minimally invasive and alternative surgical techniques for long-term contemporary LVAD placement.
General indications and algorithm for implant
Following the LVAD support selection process, all patients are evaluated to proceed to surgery using a less invasive surgical implant technique, while alternative surgical implants are reserved for high-risk patients with distinct features such as severe aortic calcification and multiple prior sternotomies (more than two). To select the appropriate implant strategy during our evaluation process, we carefully review baseline pulmonary function test results and non-contrast chest computed tomography results to evaluate the physiologic risks associated with a left thoracotomy and anatomic positioning of the ascending aorta. For patients with chronic obstructive lung disease, careful evaluation of the capacity of postoperative pulmonary recovery from a thoracotomy is performed. For sternal-sparing intercostal outflow graft (OG) approaches, a right-sided curvature of the ascending aorta is preferable. In patients with multiple previous proximal coronary artery bypasses, the length of the ascending aorta is assessed, along with the patency of grafts on preoperative angiogram, and balanced with the challenges of a less invasive approach for OG anastomosis. For patients with a planned descending aorta or left subclavian anastomosis, we routinely extend our evaluation to include careful assessment of arterial calcifications.
Preoperative assessment of contraindications
All patients considered for minimally invasive and alternative approaches undergo extensive multidisciplinary evaluation of preoperative LVAD transthoracic echocardiography (TTE) to assess chamber size, presence of significant valvular anomalies and right ventricular (RV) size and function assessment (13). The TTE provides critical information to determine the need for concomitant surgical procedures such as aortic or tricuspid valve repair or patent foramen ovale (PFO) closure at the time of LVAD implantation. Patients with more than mild aortic insufficiency (AI), previous mechanical aortic valve replacement, more than moderate tricuspid regurgitation, and/or significant mitral stenosis are preferably approached through a standard on-pump midline sternotomy incision. Additionally, preoperative functional RV assessment is vital to determine a patient’s risk of developing postoperative RV failure. For patients with moderate to severe RV dysfunction and no other valvular anomalies, we prefer to use a minimally invasive (ideally off-pump) approach, keeping the pericardium closed to protect the RV from acute unrestricted dilatation after implant (14). Our initial unpublished experience suggests a potential RV protective benefit from a minimally invasive and off-pump strategy versus a standard on-pump implantation technique. For patients with a documented preoperative LV thrombus, an on-pump minimally invasive strategy is preferred to clearly visualize the LV apex. We generally do not close a PFO unless it becomes clinically significant after implantation. If significant, percutaneous closure of the PFO using an Amplatzer occluding device is utilized. Preoperative imaging with computed tomography and vascular ultrasound studies can identify patients who have severe aortic root calcification, abdominal or descending aortic aneurysm or peripheral vascular disease, which facilitates planning for the OG anastomotic site (ascending aorta, descending aorta or subclavian artery).
Anesthesia
Based on the degree of pulmonary disease, patients considered for off-pump approaches are carefully evaluated for the need of a double-lumen intubation. In patients with moderate to severe lung disease undergoing an off-pump implant, the need to repetitively stop ventilation can alter oxygenation capacity and challenge pulmonary recovery after implant. Before opening the pericardium and manipulating the LV apex for inflow placement in patients undergoing off-pump implants, we prophylactically administer 4 g of magnesium and 100 mg of lidocaine to decrease LV arrhythmogenicity and allow proper placement of the inflow ring.
Rapid ventricular pacing has been used to temporarily reduce LV ejection during off-pump LVAD insertion (10,15). Our technique involves administration of an intravenous bolus of 30 mg of adenosine to induce a short bradycardic arrest during off-pump LVAD placement. Adenosine-induced asystole renders the left ventricle immobile, making it less technically challenging for the surgeon to place the inflow cannula in the anatomically optimal LV apical position. It further reduces blood loss by reducing both the volume of blood ejected from the heart during LVAD implant (reduction in blood pressure) and increasing time between heartbeats. We have further observed that an additional benefit of this method is adenosine-mediated pulmonary vasodilatation, which may reduce pulmonary pressures and protect the RV function (16,17). Lastly, adenosine has an extremely limited half-life which minimizes the duration and potential deleterious hemodynamic effects of asystole induction (18,19).
Surgical approaches
HeartWare ventricular assist device (HVAD)
Minimally invasive on-pump approach
Upper-hemisternotomy for outflow graft (OG) placement
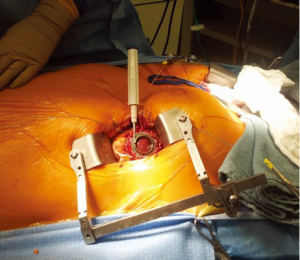
Our most commonly used on-pump minimally invasive strategy involves a less invasive modified approach as already described by Schmitto et al. (5). Similarly, a 6 cm left anterior thoracotomy is combined with a 4cm upper hemi-sternotomy for OG placement (Figure 1). Cardiopulmonary bypass (CPB) is established after off-pump placement of the LV apex sewing ring, and tunneling of the driveline to the right upper quadrant is generally performed using a 1 cm counter substernal mid-line incision. For the majority of patients, the OG is tunneled within the pericardium and anastomosed on-pump end-to-side to the proximal ascending aorta. For patients with a previous sternotomy, the HVAD OG is connected to the ascending aorta after tunneling the graft through the anterior mediastinum.
Sternal-sparing intercostal outflow graft (OG) implants
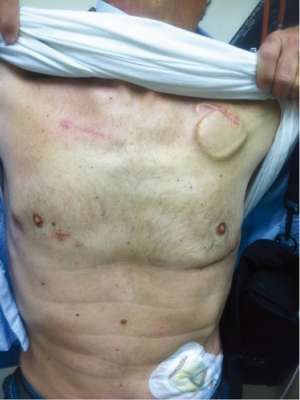
In patients with appropriate right-sided curvature positioning of the ascending aorta (based on preoperative evaluation with chest computed tomography), a second intercostal incision is utilized to create a surgical window to gain access to the aorta and perform the OG anastomosis (Figure 2). This technique is preferentially utilized in bridged patients to spare the sternal incision for heart transplantation. This approach is particularly appealing in patients with a history of coronary artery bypass surgery, where patency of previous grafts and resternotomy can represent a significant reoperative challenge.
Minimally invasive off-pump strategy
All patients implanted with an off-pump strategy are approached through a left anterior thoracotomy and an upper hemi-sternotomy. A modified non-fibrillatory technique is used for inflow cannula placement. After setting the permanent pacemaker to a backup rate of 40 beats per minute, a 30 mg bolus of adenosine is given to induce a brief bradycardic asystole, during which the LV apex is quickly incised to secure the LV coring tool. Following a brief period of recovery, a similar second bolus of adenosine is given to complete LV coring and lock the HVAD to its final position. About half of the outflow band relief is removed, leaving a shorter length to allow an easier mediastinal course and placement of the ouflow graft. The ouflow graft is tunneled similarly to the on-pump group, but anastomosed off-pump end-to-side to the ascending aorta.
Single left thoracotomy incision with descending aorta anastomosis for high-risk patients
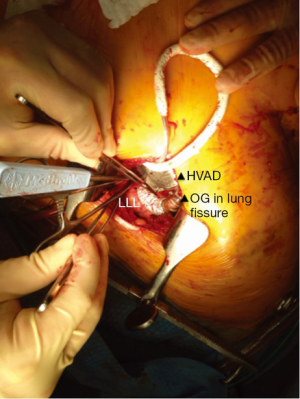
For this approach, we use a double-lumen intubation strategy to facilitate lung retraction. The femoral vessels are exposed for CPB if needed. Surface echocardiography is used to enter the left pleural space and expose the LV apex. Lung adhesions are dissected if needed, and the inferior pulmonary ligament is freed to optimize exposure. Ring placement position is confirmed utilizing TEE; if needed, a guidewire is advanced through the LV apex for ring position confirmation. Before heparin is given, the driveline is tunneled in position on the right using a 1 cm counter subxiphoid incision. For on-pump implant, the patient is heparinized, the femoral vessels are cannulated, and CPB is initiated. For the off-pump approach, we percutaneously wire both femoral vessels to get immediate access for CPB if needed, given the single incision nature of the approach. The HVAD sewing ring position is confirmed with TEE, and the apical sewing ring portion of the HVAD inflow is generally secured in place utilizing Ethibond 2-0 sutures with felts. After coring the LV, the HVAD is secured in place. Our off-pump approach is similar as outlined previously. The OG is measured and deaired. A loop is deliberately created to bury the OG in the left pulmonary fissure, creating a straight but gentle loop to the descending aorta. An off-pump end-to-side anastomosis using a HeartPort assisted technique is then performed on the descending aorta using a partial crossclamp, close to the vicinity of the junction of the diaphragm (Figure 3). The graft is de-aired with a deairing needle and carbon dioxide (CO2) is utilized throughout the procedure.
Left subclavian OG anastomosis has been increasingly utilized for specific patient situations when OG anastomosis to the ascending or the descending aorta is prohibited. Despite the theoretical risk associated with increased left arm perfusion and subsequent arm hyperemia, this technique represents a valuable option for this high-risk group of patients. We found that a progressive step-by-step increase in pump speed over a few days may allow gradual distal adaptation to the increased pump flow and decrease risks of left arm hyperperfusion.
HeartMate II left ventricular assist device (LVAD)
Minimally invasive access for HeartMate II LVAD implant is technically more challenging, as the larger design of the pump requires creation of a pump pocket for appropriate pump placement. Our strategy for minimally invasive implants employs a 4 cm subxiphoid incision to allow the creation of a large pump pocket. The abdominal rectus transversalis fascia is opened and dissected laterally to created a plane above the preperitoneal fat. The dissection is further extended laterally on the left by dividing diaphragmatic attachments and extending the created space to the left pleural cavity. Using surface TTE, a 6 cm left anterior thoracotomy is performed to expose the LV apex. CPB is initiated using femoral access and coring is performed under TEE guidance. The pump sewing ring is secured in place facing the mitral valve. The entire pump is then connected with the OG after filling with saline to avoid air accumulation. The bend relief and its connector are then connected to complete assembly of the pump. The pump is placed in the previously created pocket and the OG is tunneled in the right pleural space to the ascending aorta using the subxiphoid incision. A second intercostal incision is then performed to create a surgical window, before a 20 mm ringed Goretex graft is slid over the ouflow graft and the anastomosis is performed end-to-side to the ascending aorta. The driveline and OG are finally carefully buried below the abdominal rectus transversalis fascia before closure of the subxiphoid incision to avoid infection.
Postoperative considerations
Patients are typically extubated within 24 hours after surgery in the cardiovascular intensive care unit (CVICU). Postoperative anticoagulation and antiplatelet therapy is protocol driven according to device type. Aspirin 325 mg (enteric-coated) is given daily beginning on postoperative day (POD) 1 for both devices. For HM-II devices only, dipyridamole 75 mg, four times daily, in addition to aspirin is administered beginning on POD 1 for one month. Coumadin (INR goal 2.0-3.0) is initiated beginning on POD 2 for both devices. Postoperative heparin infusions are not used unless INR goal is not reached POD 5. Goal directed medical therapy for end-stage HF is added postoperatively according to national guidelines (20,21).
Comments
Ventricular assist device surgery has become an integral procedure for the treatment of terminal HF (22). Two dominant CF-LVAD types, the HVAD and the HM-II, have seen a dramatic increase in utilization, with more than 2,500 HVAD pumps placed and more than 14,000 HM-II implants worldwide (23). The surgical approach for CF-LVAD placement has traditionally been a midline sternotomy using full CPB support (24). Although this approach has been successful, a standard midline sternotomy may increase the risk of postoperative bleeding (25), infection (26) and sternal non-union events. Furthermore, opening the pericardium in patients undergoing LVAD implant may be associated with geometric RV changes and alteration of the RV pressure-volume relationship beyond the anticipated RV geometric changes from LVAD induced ventricular septal changes (13). Less invasive approaches in cardiac surgery were developed with the goal of reducing CPB time and operative duration, minimizing perioperative blood loss, protecting cardiac structures from multiple re-entries, and preserving biventricular geometry (27).
Potential implications of a less invasive strategy
Minimally invasive approaches to cardiac surgery have been used for mitral and aortic valve surgeries, with comparable and occasionally improved outcomes to conventional surgical approaches (27). Similar mortality rates (28), shorter intensive care unit/hospital stays (29), overall lower costs (30), decreased post operative bleeding (25,31) and improved cosmetic results (32) have been demonstrated for less invasive surgical strategies. It is conceivable that some of these advantages could be seen with minimally invasive, off-pump, and single-incision descending OG anastomosis LVAD surgeries. In bridge-to-transplantation candidates, avoiding a full sternotomy during LVAD implantation can make the subsequent LVAD explantation and heart transplantation technically less challenging by minimizing adhesion takedown and allowing easier identification of dissection planes.
Disadvantages of less invasive strategies, however, need to be acknowledged. Given the smaller nature of thoracotomy incisions, direct access to the left ventricular apex may be technically more challenging and result in malalignment of the inflow cannula. We have found that the use of a surface TTE by the surgeon before the thoracotomy incision is made is beneficial in identifying the left ventricular apex prior to performing the left thoracotomy. This assures ideal anatomic position for the thoracotomy. A hemi-sternotomy incision also results in limited exposure of the ascending aorta and can be technically challenging if there are complications with the OG anastomosis, such as bleeding from the anastomosis site, rotation during tunneling and subsequent pump OG occlusion, or emergent need to go on CPB.
Off-pump versus on-pump
Activation of the systemic inflammatory response due to CPB and associated deleterious effects on the coagulation system have been well documented in the literature (33). Fibrinolysis, platelet sequestration and degradation of coagulation factors are some of the negative effects of the CPB machine (34-36). Although long term benefits of off-pump versus on-pump coronary artery bypass surgery are controversial, the short term benefits of an off-pump strategy to reduce blood-product transfusion, reoperation for perioperative bleeding, acute kidney injury and respiratory complications have been demonstrated in a large randomized study (37). Minimizing blood product transfusions and reducing exposure to blood antigens decreases the risk of recipient sensitization, thus preserving donor pool availability for bridge-to-transplantation candidates undergoing LVAD implantation. Despite no statistically significant differences in blood products requirements at our institution (unpublished data), we believe larger studies will be adequately powered to demonstrate advantages of the off-pump approach in regards to this important endpoint.
There are circumstances where an off-pump LVAD placement is not appropriate. These include patients who require concomitant surgery for left ventricular or atrial appendage clot and patients who have significant AI, mitral stenosis, or severe tricuspid regurgitation.
Descending aortic graft anastomosis
When implanting an LVAD using a left thoracotomy approach, anastomosis of the OG can be made to the ascending aorta or to the descending aorta (4,12). Successful descending aortic anastomosis using the HVAD and HM-II OGs have been described (4,12). Detailed flow analysis from CF-LVAD patients with ascending and descending aortic anastomosis revealed nearly identical waveforms and flow rates, suggesting that descending aortic anastomosis is a potential comparable alternative option in patients receiving left thoracotomy LVAD placement (4,38). Despite demonstration of similar flow patterns in patients with descending aortic anastomosis, we believe that it is critical to adjust the pump speed in order to leave some aortic valve opening (at least 1:3) to allow aortic root washout and to decrease the chance of stagnant blood and subsequent clot formation. We recommend adjusting the pump speed to provide adequate LV ejection and antegrade flow across the aortic valve to minimize the risk of thromboembolic complications.
Conclusions
Minimally invasive and alternative surgical techniques for long-term contemporary LVAD placement are technically feasible and reproducible with contemporary HVAD and HM-II devices. We believe that ongoing surgical implant innovations occurring at the bench and bedside, fueled by collaborative discussions between high-volume institutions and research partnerships with device companies, will continue to develop novel surgical options for CF-LVAD implantation.
Less surgically invasive approaches also promise the possibility of increasing the number of high-risk surgical patients who could benefit from CF-LVAD therapies. Further large scale collaborative and randomized studies are needed to clarify the potential advantages and disadvantages of these novel techniques on CF-LVAD patient outcomes.
Acknowledgements
We would like to acknowledge Dr. Martin Strüber (Leipzig, Germany) and Dr. Jan Schmitto (Hannover, Germany) for allowing us to visit their centers and develop our strategy for less invasive and off-pump HVAD implants.
Dr. Maltais is a paid educator for Heartware.
Disclosure: The authors declare no conflict of interest.
References
- Stehlik J, Hosenpud JD, Edwards LB, et al. ISHLT International Registry for Heart and Lung Transplantation--into the fourth decade, from strength to strength. J Heart Lung Transplant 2013;32:941-50. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant 2013;32:141-56. [PubMed]
- Umakanthan R, Haglund NA, Stulak JM, et al. Left thoracotomy HeartWare implantation with outflow graft anastomosis to the descending aorta: a simplified bridge for patients with multiple previous sternotomies. ASAIO J 2013;59:664-7. [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [PubMed]
- Sabashnikov A, Mohite PN, Simon AR, et al. HeartWare miniaturized intrapericardial ventricular assist device: advantages and adverse events in comparison to contemporary devices. Expert Rev Med Devices 2013;10:441-52. [PubMed]
- Gregoric ID, La Francesca S, Myers T, et al. A less invasive approach to axial flow pump insertion. J Heart Lung Transplant 2008;27:423-6. [PubMed]
- Gregoric ID, Cohn WE, Frazier OH. Diaphragmatic implantation of the HeartWare ventricular assist device. J Heart Lung Transplant 2011;30:467-70. [PubMed]
- Anyanwu AC. Technique for less invasive implantation of Heartmate II left ventricular assist device without median sternotomy. Semin Thorac Cardiovasc Surg 2011;23:241-4. [PubMed]
- Cheung A, Lamarche Y, Kaan A, et al. Off-pump implantation of the HeartWare HVAD left ventricular assist device through minimally invasive incisions. Ann Thorac Surg 2011;91:1294-6. [PubMed]
- García Sáez D, Mohite PN, Zych B, et al. Minimally invasive access for off-pump HeartWare left ventricular assist device explantation. Interact Cardiovasc Thorac Surg 2013;17:581-2. [PubMed]
- Riebandt J, Sandner S, Mahr S, et al. Minimally invasive thoratec Heartmate II implantation in the setting of severe thoracic aortic calcification. Ann Thorac Surg 2013;96:1094-6. [PubMed]
- Topilsky Y, Hasin T, Oh JK, et al. Echocardiographic variables after left ventricular assist device implantation associated with adverse outcome. Circ Cardiovasc Imaging 2011;4:648-61. [PubMed]
- Dell’Italia LJ. Anatomy and physiology of the right ventricle. Cardiol Clin 2012;30:167-87. [PubMed]
- Centofanti P, La Torre M, Attisani M, et al. Rapid pacing for the off-pump insertion of the Jarvik left ventricular assist device. Ann Thorac Surg 2011;92:1536-8. [PubMed]
- Pelleg A, Porter RS. The pharmacology of adenosine. Pharmacotherapy 1990;10:157-74. [PubMed]
- Galiè N, Ussia G, Passarelli P, et al. Role of pharmacologic tests in the treatment of primary pulmonary hypertension. Am J Cardiol 1995;75:55A-62A. [PubMed]
- Reeves JT, Groves BM, Weir EK. Adenosine and selective reduction of pulmonary vascular resistance in primary pulmonary hypertension. Circulation 1991;84:1437-9. [PubMed]
- Nootens M, Schrader B, Kaufmann E, et al. Comparative acute effects of adenosine and prostacyclin in primary pulmonary hypertension. Chest 1995;107:54-7. [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- Molina EJ, Boyce SW. Current status of left ventricular assist device technology. Semin Thorac Cardiovasc Surg 2013;25:56-63. [PubMed]
- Slaughter MS. Implantation of the HeartWare left ventricular assist device. Semin Thorac Cardiovasc Surg 2011;23:245-7. [PubMed]
- Dogan S, Aybek T, Risteski PS, et al. Minimally invasive port access versus conventional mitral valve surgery: prospective randomized study. Ann Thorac Surg 2005;79:492-8. [PubMed]
- Grossi EA, Galloway AC, Ribakove GH, et al. Minimally invasive port access surgery reduces operative morbidity for valve replacement in the elderly. Heart Surg Forum 1999;2:212-5. [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive aortic valve replacement. Semin Thorac Cardiovasc Surg 1997;9:331-6. [PubMed]
- Asher CR, DiMengo JM, Arheart KL, et al. Atrial fibrillation early postoperatively following minimally invasive cardiac valvular surgery. Am J Cardiol 1999;84:744-7, A9.
- Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544-52. [PubMed]
- Grossi EA, Galloway AC, Ribakove GH, et al. Impact of minimally invasive valvular heart surgery: a case-control study. Ann Thorac Surg 2001;71:807-10. [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. Mitral valve surgery can now routinely be performed endoscopically. Circulation 2003;108 Suppl 1:II48-54. [PubMed]
- Clive Landis R, Murkin JM, Stump DA, et al. Consensus statement: minimal criteria for reporting the systemic inflammatory response to cardiopulmonary bypass. Heart Surg Forum 2010;13:E116-23. [PubMed]
- Spiess BD. Transfusion of blood products affects outcome in cardiac surgery. Semin Cardiothorac Vasc Anesth 2004;8:267-81. [PubMed]
- Momeni M, Carlier C, Baele P, et al. Fibrinogen concentration significantly decreases after on-pump versus off-pump coronary artery bypass surgery: a systematic point-of-care ROTEM analysis. J Cardiothorac Vasc Anesth 2013;27:5-11. [PubMed]
- Belhaj A. Actual knowledge of systemic inflammation reaction during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov 2012;7:165-9. [PubMed]
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012;366:1489-97. [PubMed]
- Bonnemain J, Malossi AC, Lesinigo M, et al. Numerical simulation of left ventricular assist device implantations: comparing the ascending and the descending aorta cannulations. Med Eng Phys 2013;35:1465-75. [PubMed]





