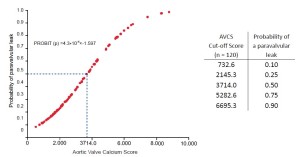
Aortic valve calcium load before TAVI: Is it important?
Cardiac surgeons were trained for decades to avoid paravalvular leakage as a major complication after aortic valve replacement. In contrast, with the widespread use of transcatheter aortic valve implantation techniques, paravalvular leaks are now considered as an acceptable outcome without serious consequences by some investigators. This might be true for trivial or mild paravalvular incompetence in patients within their last decade of life. However, with a more liberal TAVI indication, i.e. TAVI expansion to operable patients, even mild paravalvular leaks might be a matter of concern. For the first time, on the basis of a prospective, multicenter, randomized clinical trial, even mild paravalvular leaks have been associated with an increased long-term mortality (1). In addition, a recent study from the German transcatheter aortic valve registry has revealed that patients with paravalvular leaks are not just sicker in general, but are prone to a considerably higher in-hospital mortality (2). Thus, Aortic Valve Calcium Scoring (AVCS) pre- TAVI might be important with regard to outcome and paravalvular leaks.
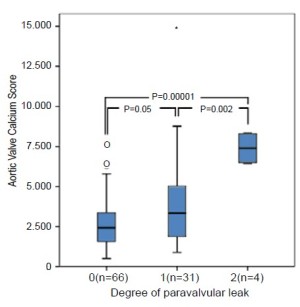
A recent study at the Heart Center Leipzig demonstrated a significant association between native aortic valve calcification and paravalvular leak in 120 patients [odds ratio (OR; per AVCS of 1,000), 11.38; 95% confidence interval (CI), 2.33-55.53; P=0.001, (3)]. Echocardiography (ECG)-gated cardiac computed tomography quantified the amount of calcification of the aortic valve leaflets using a scoring system analogous to the Agatston calcium scoring of coronary arteries [Aortic Valve Calcium Scoring (AVCS), Figure 1]. Paravalvular leaks were assessed and quantified intra-operatively by transoesophageal echocardiography (TEE) and root angiography. All valves were implanted successfully. The mean AVCS in patients without paravalvular leaks (n=66) was 2704±1510; with mild paravalvular leaks (n=31) was 3804±2739 (P=0.05); and with moderate paravalvular leaks (n=4) was 7387±1044 (P=0.002) (Table 1 and Figure 2). Consistent with this study, two further studies documented comparable results using the CoreValve ReValving system (4,5). Wood et al. (6) in contrast, did not find an association between paravalvular leaks and the degree of calcification, which was probably a result of the small study population.
| Table 1 Preoperative transesophageal echocardiography results and mean Aortic Valve Calcium Score’s (AVCS) for the aortic valve, cusps and commissures depending on the presence of a paravalvular leak | |||||||||||||||
| AVCS | No paravalvular leak* | Paravalvular leak* | P-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aortic valve | 2694±1528 | 4153±479 | 0.006 | ||||||||||||
| Right coronary cusp | 811±542 | 1189±882 | 0.025 | ||||||||||||
| Left coronary cusp | 919±644 | 1669±1514 | 0.001 | ||||||||||||
| Non-coronary cusp | 1013±671 | 1281±750 | 0.053 | ||||||||||||
| Right-left-coronary commissure | 782±554 | 1295±1071 | 0.010 | ||||||||||||
| Left-non-coronary commissure | 1049±656 | 1589±1104 | 0.012 | ||||||||||||
| Non-right-coronary commissure | 918±560 | 1258±941 | 0.110 | ||||||||||||
| Aortic Valve Calcium Score’s (AVCS) for the aortic valve, cusps and commissures depending on the presence of a paravalvular leak. *: confirmed by intraoperative transesophageal echocardiography (TEE) and root angiography | |||||||||||||||

When analysing the localization of paravalvular leaks based on TEE, there was also a significant relation to the AVCS in each separate cusp or commissure (Table 2). A significant association was found for the right and left coronary cusp, and for the right-left and left-non-coronary commissure. This association, however, failed to reach statistical significance for the non-right-coronary commissure and the non-coronary cusp. One possible reason for this might be the intrinsic weakness and elasticity of the annulus in this area, leading to an anatomic predisposition to paravalvular leaks (7).
| Table 2 Association of Aortic Valve Calcium Score (AVCS), localization and degree of paravalvular leaks | |||||||||||||||
| Impact of aortic valve calcification on paravalvular leaks | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Localization of paravalvular leak (TEE) | |||||||||||||||
| R | L | N | |||||||||||||
| Calcium Score cusp (CT) | R | 5.64 |
1.84 |
1.03 |
|||||||||||
| L | 1.94 |
5.43 |
1.61 |
||||||||||||
| N | 1.85 |
3.59 |
1.35 |
||||||||||||
| RL | LN | NR | |||||||||||||
| commissure | RL | 4.88 |
2.70 |
1.02 |
|||||||||||
| LN | 1.66 |
5.03 |
1.24 |
||||||||||||
| NR | 1.27 |
2.25 |
1.37 |
||||||||||||
| Data are presented as odds ratio per AVCS of 1000 and corresponding P-values. *confirmed by intraoperative transesophageal echocardiography. Abbreviations: R, right coronary; L, left coronary; N, non-coronary; RL, right-left coronary; LN, left-non coronary; NR, non-right coronary | |||||||||||||||
There is well-accepted agreement, that valve calcification is a surrogate marker for the biological age and general morbidity of an individual patient. Temporary haemodialysis as well as ventilation time were both associated with a significantly higher AVCS (Table 3). More frequently increased AVCS values were observed in those with impaired respiratory function and renal disease, thus, suggesting that these patients are at a higher risk for postprocedural secondary complications and a longer in-hospital stay. Based on our experience, however, the AVCS was not a predictor of 30-day mortality, major cardiac events and stroke.
| Table 3 Impact of aortic valve calcification on outcome | |||||||||||||||
| p-value | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paravalvular leak* | [2.33;55.53] | 11.38 | 0.001 | ||||||||||||
| Major cardiac event | [0.68;1.25] | 0.92 | 0.57 | ||||||||||||
| Stroke | [0.41;1.96] | 0.90 | 0.79 | ||||||||||||
| New pacemaker-implantation | [0.85;1.89] | 1.27 | 0.26 | ||||||||||||
| Temporary hemodialysis | [0.96;14.53] | 3.73 | 0.049 | ||||||||||||
| Median ventilation time ≥ 60 h | [1.73;36.56] | 7.94 | 0.005 | ||||||||||||
| Reintubation | [0.86;9.82] | 2.90 | 0.089 | ||||||||||||
| 30-day mortality | [0.84;1.32] | 1.05 | 0.68 | ||||||||||||
| Data are presented as odds ratio per AVCS of 1,000, corresponding P-values and confidence intervalls (CI). * confirmed by postoperative transthoracic echocardiography before discharge | |||||||||||||||
In contrast, in a previously published study (8) the degree of aortic valve calcification (calcium mass-score) was a significant predictor for 30-day MACE and for 1-year mortality. Patients with severe periprocedural complications (death, acute myocardial infarction and stroke) revealed significant more aortic valve calcium than patients without any complications. By selecting a calcium mass-score threshold of 750 the authors were able to identify more than 70% of patients who suffered subsequently from MACE or died within the first year. They concluded that such a parameter had enormous value in order to better select and risk-stratify candidates for TAVI.
Cardiac computed tomography (CT) is the gold standard imaging technique for assessing aortic valve calcification. Although, the cardiac CT-based AVCS has not been not cross-validated with the intraoperative transesophageal echocardiography (TEE), TEE assessment may add further information to better understand the morphology of the native calcified valve. A recent paper by Colli et al. (9) has examined the usefulness of an echocardiographic calcification score (ECS) to predict outcomes in 103 transapical TAVI patients. The ECS, as well as the aortic commissure calcification score alone, were predictive for the development of post- TAVI aortic regurgitation. The ECS was associated with the presence of moderate paravalvular aortic regurgitation (odds ratio 8.5; 95% confidence intervall, 1.2-58.9; P=0.0001) and overall moderate aortic regurgitation (odds ratio 3.6, 95% confidence intervall, 1.2-10.4; P=0.0006). The TEE gave detailed anatomic information of the calcification patterns of the aortic valve. The echocardiographic calcification score (ECS) may be used to identify patients at high risk for the development of post-TAVI AR and may therefore support decision making in the future. The main drawback of the study from Colli et al. (9) is the retrospective nature. Prospective randomized studies will be necessary to conclusively answer the question whether preoperative AVCS should be mandatory and helpful to predict AR after TAVI.
On the other hand, a more calcified aortic root might offer superior grip and better seating in the native annulus during deployment. In a study by van Miegham et al. (10) patients with valve dislodgment had significantly less aortic root calcification using the Medtronic CoreValveTM system [median Agatston score 1,951 AU (IQR, 799-3,103) vs. 3,289 AU (IQR 2,097-4,481), P=0.016]. The incidence of valve dislodgment was three times higher when the Agatston calcification score was <2,359 AU by multi-slice computed tomography. Thus, in patients with a low AVCS the impact of aortic root calcium score on valve dislodgment seems robust and warrants further awareness among surgeons.
In summary, the AVCS identifies patients at risk for a relevant paravalvular leak. The AVCS prior to TAAVI might serve as an additional tool for surgeons to reconsider the TAVI indication and valve size to reduce the risk of paravalvular leaks (Figure 3). Clinically, we especially consider patients with a borderline risk score and a high calcium burden as ‘non-TAVI candidates’ (3). In self-expanding transcatheter heart valves which don't have the same radial forces as balloon-expandable valves, it may even play a major role, and even more impact on the postoperative result in terms of the persistence of paravalvular leaks and residual stenosis. If we can predict somehow whether these patients will have TAVI regurgitation or not, and this is one way to do it, it will in the future help us to define the best valve for a given patient and the best procedure.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95.
- Abdel-Wahab M, Zahn R, Horack M, et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart 2011;97:899-906.
- Haensig M, Lehmkuhl L, Rastan AJ, et al. Aortic valve calcium scoring is a predictor of significant paravalvular aortic insufficiency in transapical-aortic valve implantation. Eur J Cardiothorac Surg 2012;41:1234-41.
- John D, Buellesfeld L, Yuecel S, et al. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc Interv 2010;3:233-43.
- Koos R, Mahnken AH, Dohmen G, et al. Association of aortic valve calcification severity with the degree of aortic regurgitation after transcatheter aortic valve implantation. Int J Cardiol 2010;150:142-5.
- Wood DA, Tops LF, Mayo JR, et al. Role of multislice computed tomography in transcatheter aortic valve replacement. Am J Cardiol 2009;103:1295-301.
- De Cicco G, Lorusso R, Colli A, et al. Aortic valve periprosthetic leakage: anatomic observations and surgical results. Ann Thorac Surg 2005;79:1480-5.
- Leber AW, Kasel M, Ischinger T, et al. Aortic valve calcium score as a predictor for outcome after TAVI using the CoreValve revalving system. Int J Cardiol 2011 [Epub ahead of print].
- Colli A, D’Amico R, Kempfert J, et al. Transesophageal echocardiographic scoring for transcatheter aortic valve implantation: Impact of aortic cusp calcification on postoperative aortic regurgitation. J Thorac Cardiovasc Surg 2011;142:1229-35.
- Van Mieghem NM, Schultz CJ, van der Boon RM, et al. Incidence, timing, and predictors of valve dislodgment during TAVI with the medtronic corevalve system. Catheter Cardiovasc Interv 2011;79:726-32.