Transapical aortic valve implantation with anatomically oriented prostheses
IntroductionOther Section
- Introduction
- The JenaValveTM
- The Symetis AcurateTM valve
- Medtronic EngagerTM valve
- Conclusions
- Acknowledgements
- References
Transcatheter aortic valve implantation (TAVI) is an accepted treatment option for elderly high-risk patients with aortic valve stenosis (1). Obvious advantages are the avoidance of a sternotomy and cardio-pulmonary bypass. Two devices are mainly used, the SapienTM Valve (Edwards Lifesience, USA) and the CoreValveTM (Medtronic, USA). The SapienTM prosthesis is implanted either transfemorally or transapically whereas the CoreValveTM prosthesis is almost exclusively implanted transfemorally. A major concern in TAVI procedures is malpositioning of the valve prosthesis which can lead to paravalvular or transvalvular regurgitation (AR) (2), or cause coronary obstruction (CO) (3).
CO is a rare but severe complication occurring at a rate of 1 to 2% (4,5). In contrast, paravalvular regurgitation is relatively common: In the patient population from the German transcatheter aortic valve interventions registry, almost one fifth of the patients showed moderate to severe AR immediately after implantation. AR was a strong independent predictor of in hospital death but no follow-up data were published (6). The 2 year follow-up results of the Partner trial showed that moderate or severe paravalvular leakage as seen in 6.9% of patients post TAVI was associated with an increase in late mortality and directly correlated with the severity of the paravalvular leakage (7). Those results underline the importance of a perfect placement of transcatheter aortic valve prostheses.
To address those complications three devices have been launched lately: The Medtronic EngagerTM System, the Symetis AccurateTM Valve and the JenaValveTM. All three valves are designed for transapical implantation and have different mechanisms to facilitate positioning in an anatomically oriented position.
The JenaValveTMOther Section
- Introduction
- The JenaValveTM
- The Symetis AcurateTM valve
- Medtronic EngagerTM valve
- Conclusions
- Acknowledgements
- References
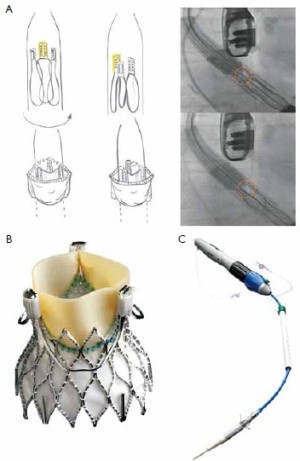
The JenaValveTM (JenaValve, Munich, Germany) consists of a self-expandable NitinolTM stent designed for subcoronary implantation. The leaflets are made of porcine root valve tissue fitted with an outer porcine pericardial skirt (Figure 1A).
Three “feelers” at the stent are placed in the sinuses of the patient’s native valve to align the commissures of the prosthesis with the commissures of the patient’s native valve. After placement in the correct position, the patient’s native valve leaflets are clipped between the feelers and the base of the prosthesis to firmly anchor the JenaValveTM in an anatomically correct position (Figure 1B). Thus, the stent design relies on axial in addition to radial fixation with a low profile to prevent coronary obstruction. The JenaValveTM is available in sizes 23, 25 and 27 mm for implantation in annuli with a diameter of 21 to 27 mm. A sheath-less 32F delivery system is used for antegrade transapical implantation. After the first-in-man implantation of the JenaValveTM in 2010 (8) CE-mark application was successful after completion of a pivotal study with 73 patients. The mean age of those patients was 83.2 years and the mean logistic EuroSCORE was 28.4. A procedural success rate of 89.6% (n=60) was achieved: 73 patients were enrolled. 67 patients were planned for implantation, but four patients were converted to conventional surgery, two patients needed a valve in valve procedure and one patient was converted to another TAVI device. Hemodynamic compromise during implant could be avoided because rapid pacing is not necessary for implantation of the JenaValveTM. 30 days mortality was 7.6%, perioperative stroke occurred in 3% and first time pacemaker implantation was necessary in 9.1% of the implanted patients. No coronary obstruction was detected and the mean gradients were decreased significantly at 30 days follow up. No or minimal paravalvular leakage was present in 86.4% of the patients and none had severe (>2+) postprocedural paravalvular aortic regurgitation (9).
The Symetis AcurateTM valveOther Section
- Introduction
- The JenaValveTM
- The Symetis AcurateTM valve
- Medtronic EngagerTM valve
- Conclusions
- Acknowledgements
- References
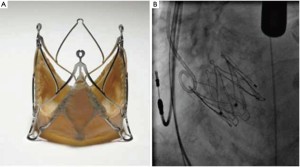
The Symetis AcurateTM (Symetis Inc., Switzerland) valve consists of a self-expanding nitinol stent designed for intra-annular, sub-coronary positioning. Three stabilization arms are connected at the stent to stabilize the valve in the ascending aorta with the intention to prevent tilting during deployment. Inside the stent, a porcine, non-coronary sinus tissue valve is mounted. The distal edge of the stent body is not covered to minimize the risk of coronary artery obstruction (Figure 2A). The ‘upper crown’ is intended to provide axial fixation and to have a tactile feedback for anatomically oriented valve positioning similar to a ‘hook concept’. The final orientation is achieved by applying slight tension on the delivery system and, thereby, on the ‘upper crown’ during the final step of implantation. Thus, independent from the operator, the device is meant to be ‘self-positioning’ (Figure 2B) in an anatomically correct rotation and in an intra-annular, subcoronary alignment of the main body. It is available in three sizes small (23 mm), medium (25 mm) and large (27 mm) and covers an aortic annulus range of 21 to 27 mm. The implantation is performed transapically with a delivery catheter, which allows for a sheathless implantation (Figure 2C). The Symetis AcurateTM transcatheter system has CE-mark since September 2011.
Kempfert and colleagues described a series of 40 patients in whom the Symetis AcurateTM valve was implanted transapically (10). Mean age of the patients was 82 years with a mean logistic EuroSCORE of 21.5% and a mean STS-Score of 9.0 %. All patients had severe aortic valve stenosis with a mean gradient of 52.6 mmHg. All valves were implanted successfully. Five patients died within the 30 days follow up period. Stroke rate was 5% and permanent pacemaker implantation was necessary in one patient. Until the 6-month follow-up, two additional patients expired (mid-term survival 82.5%), two patients had a stroke and three patients required pacemaker implantation. The valve function was stable compared to the 30 days follow-up and NYHA classification improved allowing the conclusion that the Symetis AcurateTM valve implantation resulted in good mid-term outcomes in this high risk patient population (11).
Medtronic EngagerTM valveOther Section
- Introduction
- The JenaValveTM
- The Symetis AcurateTM valve
- Medtronic EngagerTM valve
- Conclusions
- Acknowledgements
- References
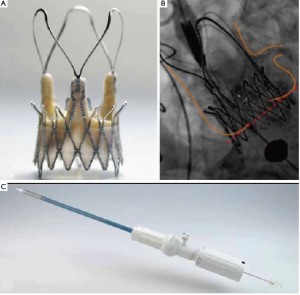
The EngagerTM Aortic Valve prosthesis (Medtronic, Inc., Minneapolis, MN, USA), consists of a self-expanding Nitinol frame. The stents consist of a main frame and a support frame. The support arms are designed to be placed into the sinuses of the aortic root to achieve a periannular, anatomically oriented positioning to minimize the risk of coronary obstruction (“rotational positioning”, Figure 3A). The main frame is sewn to a polyester sleeve. Mounted to the stent is a standard biological heart valve prosthesis composed of three leaflets, made of tissue-fixated bovine pericardium (Figure 3B). The stent design aims to minimize paravalvular leakage. The sizes 23 and 26 mm are available to fit annulus diameters of 19 to 26 mm. Implantation is performed transapically with a delivery system composed of an introducer and a flexible delivery catheter, which forms an integral unit (Figure 3C).
The predecessor of the Medtronic EngagerTM valve, the Ventor Embracer valve has been investigated in a first-inman study with thirty patients primarily (12). Mean age of the patients was 83.4 years with a logistic EuroSCORE of 23.4%. All patients had severe aortic stenosis with a mean aortic valve pressure gradient of 52.1 mmHg. All implantations were performed successfully. 29 prostheses had an anatomically correct position. 6 patients (20%) died within 30 days. Three patients required permanent pacemaker implantation and 5 patients required temporary hemodialysis (four had preexisting renal failure). Type A dissection was diagnosed in 4 patients (13%). Three patients developed the dissection during the operation. The uncovered prosthetic commissural posts of the crimped prosthesis in combination with a rigid delivery system, interacting with the aortic wall while advancing the valve caused aortic injury. Thus, the delivery system and the valve were completely redesigned. For the EngagerTM vavle in the current version, the prosthesis is completely covered and the delivery system is flexible with a soft tip. A feasibility study was conducted with the new EngagerTM transcatheter system (13). Ten patients have been implanted with a mean age of 82.5 years and a mean logistic EuroSCORE of 24.6%. All patients were implanted successfully with anatomically correct positioning. The invasively measured gradient was 7.1 mmHg after implantation and no paravalvular leak higher then grade I was observed. One implanted patient (logistic EuroSCORE 48.9%) expired at POD 23 in multi organ failure. At 30 days follow-up mean gradient measured by TTE was 15.6 mmHg and no more then trivial trans- or paravalvular leakage was assessed. Two patients required permanent pacemaker implantation. No dissection, coronary obstructions, undesired interferences with the mitral valve or other device related complications were observed (Table 1). At the moment patients are enrolled in a multicenter pivotal trial to receive CE-mark for the system.
| Table 1 Results of the EngagerTM Feasibility Trial; *: at 30 days follow-up; **: data for one patient is not available | |
| Accurate device placement (patients) | 10 (100%) |
|---|---|
| Used prosthesis size (patients) |
4 (40%) |
| Skin-to-skin time (min.) | 94.5±16.7 |
| Contrast medium volume (mL) | 103±32 |
| Fluoroscopy time (min) | 8.6±2.5 |
| Aortic Dissection* | 0 |
| Mean aortic valve gradient (mmHg)* | 15.6±4.0 |
| Mean LVOT gradient (mmHg)* | 2.2±1.0 |
| Peak aortic valve velocity (m/s)* | 2.7±0.5 |
| Peak LVOT velocity (m/s)* | 1.0±0.2 |
| Velocity time integral (cm)* | 20.4±5.1 |
| Aortic regurgitation*,** |
- |
| New Pacemaker Implantation* | 2 |
ConclusionsOther Section
- Introduction
- The JenaValveTM
- The Symetis AcurateTM valve
- Medtronic EngagerTM valve
- Conclusions
- Acknowledgements
- References
Three new transcatheter aortic valve replacement systems have been launched lately to face the problem of coronary obstruction and paravalvular leakage, which is an independent predictor of late mortality after TAVI. The results of the described trials show, that anatomically oriented positioning of those prosthesis might be an advantage in regards to avoiding coronary obstruction and paravalvular leakage. However, these trials only included small numbers of patients and no long-term follow up data are available. Larger trials with longer follow-up periods have to be performed to prove the efficacy of anatomically oriented transcatheter aortic valve implantation systems.
AcknowledgementsOther Section
- Introduction
- The JenaValveTM
- The Symetis AcurateTM valve
- Medtronic EngagerTM valve
- Conclusions
- Acknowledgements
- References
Disclosure: The authors declare no conflict of interest.
ReferencesOther Section
- Introduction
- The JenaValveTM
- The Symetis AcurateTM valve
- Medtronic EngagerTM valve
- Conclusions
- Acknowledgements
- References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
- Clavel MA, Dumont E, Pibarot P, et al. Severe valvular regurgitation and late prosthesis embolization after percutaneous aortic valve implantation. Ann Thorac Surg 2009;87:618-21.
- Kapadia SR, Svensson L, Tuzcu EM. Successful percutaneous management of left main trunk occlusion during percutaneous aortic valve replacement. Catheter Cardiovasc Interv 2009;73:966-72.
- Kodali SK, O’Neill WW, Moses JW, et al. Early and late (one year) outcomes following transcatheter aortic valve implantation in patients with severe aortic stenosis (from the United States REVIVAL trial). Am J Cardiol 2011;107:1058-64.
- Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter Aortic Valve Implantation for the Treatment of Severe Symptomatic Aortic Stenosis in Patients at Very High or Prohibitive Surgical Risk. J Am Coll Cardiol 2010;55:1080-90.
- Abdel-Wahab M, Zahn R, Horack M, et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart 2011;97:899-906.
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95.
- Kempfert J, Rastan AJ, Mohr FW, et al. A new selfexpanding transcatheter aortic valve for transapical implantation - first in man implantation of the JenaValve™. Eur J Cardiothorac Surg 2011;40:761-3.
- Treede H, Mohr FW, Baldus S, et al. Transapical transcatheter aortic valve implantation using the JenaValve™ system: acute and 30-day results of the multicentre CE-mark study. Eur J Cardiothorac Surg 2012;41:e131-8.
- Kempfert J, Rastan AJ, Beyersdorf F, et al. Trans-apical aortic valve implantation using a new self-expandable bioprosthesis: initial outcomes. Eur J Cardiothorac Surg 2011;40:1114-9.
- Kempfert J, Treede H, Rastan AJ, et al. Transapical aortic valve implantation using a new self-expandable bioprosthesis (ACURATE TA™): 6-month outcomes. Eur J Cardiothorac Surg 2012. [Epub ahead of print].
- Falk V, Walther T, Schwammenthal E, et al. Transapical aortic valve implantation with a self-expanding anatomically oriented valve. Eur Heart J 2011;32:878-87.
- Sündermann SH, Grünenfelder J, Corti R. et al. Feasibility of the Engager™ aortic transcatheter valve system using a flexible over-the-wire design. Eur J Cardiothorac Surg 2012. [Epub ahead of print].