Durability of continuous-flow left ventricular assist devices: a systematic review
Introduction
Left ventricular assist devices (LVADs) are emerging as an increasingly viable alternative therapy for heart failure, either as a bridge to heart transplantation (BTT) or permanent destination therapy (DT) (1). The latter has become increasingly popular in recent years, in the face of a donor organ shortage and a rise in elderly patients ineligible for heart transplants (1). Key advances in LVAD technology continue to improve long-term outcomes and quality of life, and include the emergence of continuous-flow devices (CF-LVADs) that are smaller and more durable than older pulsatile models (2-4).
As the average duration of long-term mechanical support increases, device durability (as measured by the rate of device failure over time) becomes an increasingly critical contributor to patient survival, morbidity, and overall quality of life (5). In the most severe cases, device failure can be fatal. In other circumstances, the subsequent requirement for pump replacement incurs additional healthcare costs and exposes the patient to potentially serious complications such as thrombosis and bleeding (6). Despite improvements in the durability of CF-LVADs, continuing causes of device failure include mechanical problems such as drive unit failure and percutaneous lead damage, as well as conditions such as device thrombosis, hemolysis and infection (6-8).
The present systematic review therefore aimed to assess the long-term durability of current CF-LVADs, as defined by rates of device exchange and death related to pump failure, and evaluate the major causes of device failure. Secondary endpoints included long-term survival without the need for device replacement.
Materials and methods
Literature search strategy
Six electronic databases, including MEDLINE, EMBASE, PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), and Database of Abstracts of Reviews of Effects (DARE), were searched from their dates of inception to August 2014. To maximize the sensitivity of the search strategy, we combined the terms: “heart-assist devices” or “LVAD” or “left ventricular assist” or “assisted circulation” AND “pump failure” or “device failure” or “equipment failure” or “exchange” or “replacement” as either keywords or MeSH terms. The references of retrieved articles were also reviewed in order to identify further relevant studies.
Selection criteria
Eligible studies included clinical trials in which at least 20 adult patients received CF-LVADs as either bridge to transplantation or DT for heart failure. Studies that did not report the incidence of device failure were excluded. When institutions published duplicate studies with cumulative sample sizes or increasing lengths of follow-up, only the most recent reports were included. All studies were limited to those published in the English language. Case reports, conference abstracts, editorials, and expert opinions were excluded. Review articles were additionally excluded, due to the potential for duplication of studies.
Data extraction and critical appraisal
Data were extracted from article texts, tables and figures for the following: study characteristics (study period, institution, types of LVAD used), patient demographics, mean duration of LVAD support, total and long-term rates of device failure, duration to time of LVAD failure or replacement, etiology of LVAD failure, and long-term rates of transplantation and survival without device failure. LVAD failure was defined as any device malfunction necessitating device exchange or explantation, or causing patient mortality. Two investigators independently reviewed each article (A.X. and K.P.), and discrepancies between the reviewers were resolved by discussion to reach a consensus. The final results were reviewed by the senior investigator (T.D.Y.).
Statistical analysis
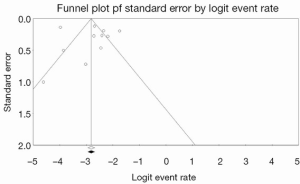
Conventional descriptive statistics were used to summarize the baseline demographics of included patients. Data were presented as raw numbers, percentages, or means with standard deviations unless otherwise indicated. Pooled averages were calculated for outcomes reported in at least three of the included studies. When not explicitly reported in the article text, patient survival data and rates of device failure were reconstructed from digitized Kaplan-Meier curves using the web-based software program, WebPlotDigitizer v3.3. The studies were assessed for publication bias by constructing funnel plots and using Egger’s linear regression method (9). If studies appeared to be missing in areas of low statistical significance, publication bias was a more likely cause of funnel asymmetry. A contour-enhanced funnel plot was performed to aid interpretation of the funnel plot, and trim-and-fill analysis was used to investigate possible asymmetry.
Results
Quantity and quality of evidence
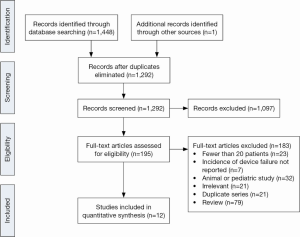
In total, 1,448 records were identified through electronic searches of the six databases (Figure 1). After screening the articles based on abstract content, 195 full-text publications were assessed according to the selection criteria, and the reference lists were also searched to identify additional relevant articles. Twelve relevant studies were included in the current review, all of which were observational and retrospective (Table 1) (6,10-20). Where studies presented separate data for patients receiving continuous- and pulsatile-flow LVADs (6,17,20), only the outcomes for CF-LVADs were included for analysis.
Full table
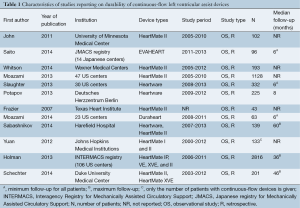
The median sample size was 166 (range, 43-2,816). Three large US multicenter studies were included (12,13,18), as well as data from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry of mechanical assist devices in US patients (21). Although this may have resulted in some duplication of patients, this was thought to be considerably outweighed by the unique data contributed by each study. Furthermore, two studies included patients as young as 15 years old (10,22). However, these studies were still included for analysis as young patients only represented a small proportion of the total sample size.
In one study, LVAD failure was defined as malfunction leading to device exchange or device-related death (6). In the remaining studies, device failure was defined as malfunction necessitating explantation or replacement (10-20).
Demographics and LVAD types
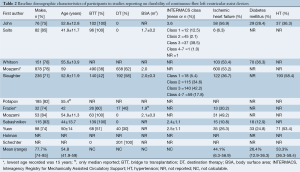
The baseline demographics are summarized in Table 2. A total of 5,471 patients were included in the present analysis. Of these, 77.7% were male, and the mean weighted age was 54.8 years. Ischemic heart disease was the etiology of cardiac failure in 44.1% of patients. On average, 26.4% of patients were diabetic and 53.3% hypertensive. The CF-LVADs used included the HeartMate II, EVAHEART, Heartware, and Duraheart devices.
Full table
Device failure
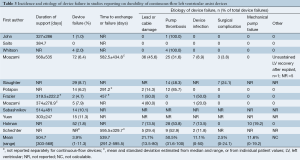
The incidence and etiology of device failure is summarized in Table 3. The mean duration of LVAD support was 504.7 days, and the weighted incidence of device failure was relatively low, at 3.9%. For patients with LVAD failure, the average time to device exchange or malfunction was 539.7 days, though this varied greatly (Table 3). The primary etiology of device failure varied considerably amongst the studies, with pump thrombosis being the most common cause overall (50.5% of total device failures). This was followed by lead or cable damage (21.7%), mechanical pump failure (11.6%), device infection (11.1%), and surgical complications associated with the LVAD implantation procedure (2.5%). Other causes of failure reported in one study included unsustained left ventricular recovery following explantation of a pre-existing LVAD (n=1) (12).
Full table
Long-term LVAD durability and survival
Data were interpolated from the competing outcomes and Kaplan-Meier curves of eight studies to approximate long-term rates of device failure and survival with the original LVAD in place (6,10-13,15,16,18). These outcomes are summarized in Table 4. Analysis using weighted means revealed rates of device failure of 0.5%, 1.8%, 2.9%, 4.5% and 6.5% at 2-, 6-, 12-, 18- and 24-months post implantation, respectively. The weighted survival with the original device in place was 90.2% at 2 months, declining to 54.4% at 12 months, and 38.7% at 24 months.

Full table
Publication bias
Inspection of the funnel plot (Figure 2) did not show significant asymmetry when overall incidence of device failure was selected as an outcome. Egger’s linear regression method suggested that publication bias was not a significant influencing factor (P=0.81520). Trim-and-fill analysis revealed that there were no missing studies, and thus no change in overall effect size was observed. These trends suggest that publication bias did not have a significant influence on the presented analysis.
Discussion
The durability and functionality of LVADs is influenced by several factors. These include implantation technique, anatomical constraints, patient complications such as infection and bleeding, treatment (including anticoagulation regimens), pump settings, and device design and manufacturer (23). While CF-LVADs have demonstrated improved durability compared to older pulsatile models in studies with up to 24 months follow-up (2-4), several causative factors have been identified that contribute to maintained rates of device failure. In the present review, the weighted overall incidence of device failure was 3.9% (1-11.3%), though this increased to 6.5% (0-9.6%) when the four studies that followed patients for up to 24 months post-implantation were considered.
The variability in device failure rates, particularly in the study by Saito et al., may be attributed to several factors (16). These include differences in study indications for device failure and different continuous flow-devices used, such as the EVAHEART and HeartMate II LVADs. The results of the current review are nonetheless consistent with previous studies, which showed that a steady minority of patients will require pump exchange (8,24,25). However, with the expected rise in LVAD usage for end-stage heart failure, this number is likely to increase, reinforcing the importance of maximizing device durability.
The most common cause of failure identified in this review was pump thrombosis, which was previously considered to be a fairly rare complication (<0.03 events per patient year) (26). In the past two years, however, data published by several US institutional studies and the INTERMACS registry has shown that the incidence of pump thrombosis has increased by as much as six-fold from 2008 to 2012 (23,27). This rate is much more variable amongst recent studies from Europe and the UK, partly due to different anticoagulation regimens and definitions of pump thrombosis (28). Nonetheless, these studies reinforce the growing significance of pump thrombosis as a risk factor for device failure and pump-related death (26). The mechanisms of this complication remain poorly understood, though decreased pump speeds and relaxed anticoagulant protocols are thought to play a role (26). In the future, strategies such as more individualized patient anticoagulation protocols may be required (12).
The second most common cause of failure identified was damage to the device cable. Potapov et al. identified the lead portion most proximal to the pump body as being a particularly vulnerable spot for cable breaks (29). Accidental pulling on the cable, weight gain leading to increased cable strain, and physically active patient lifestyles have been reported to contribute to cable fractures (12,29). Strategies to prevent accidental cable pulls have included C-shaped tunneling of the cable and looping of the driveline inside the body (29). Furthermore, several targeted design modifications have involved softer cable material and rounded mould edges in order to minimize cable strain (12). To date, these techniques have proved highly effective in reducing the incidence of cable breakage (12,29). Potential future strategies include the use of transcutaneous energy sources to remove the need for a driveline altogether (29).
Device-specific infection was another important cause of failure in this review, and can be categorized into pump and/or cannula, pocket, and percutaneous driveline exit site infections (30). Although most device infections are initially superficial, a significant proportion can evolve over months into deep infections, resulting in an increased risk of hospital readmissions, bloodstream infections, sepsis, and other serious complications (31). Furthermore, the causative pathogen in an existing device infection can also change over time, especially in the context of suppressive antibiotics and drug-resistance (31). In addition, LVAD exchange is not consistently curative in the setting of progressive device infection, and lifelong antibiotic therapy may still be required (31). Strategies to prevent infectious complications have included immobilizing the device driveline at the skin level in order to reduce traction or tearing, and thereby improve wound healing (32). Of course, eliminating the need for a driveline altogether, with a transcutaneous energy source, would similarly reduce the incidence of this complication.
In the current review, post-exchange morbidity and mortality were poorly reported and thus not included in the analysis. Studies which have examined these rates have been mostly case series, and only one of these included a matched primary implant control group for comparison (17). This trial found that patients requiring device replacement had a significantly reduced survival at 30 days (90% vs. 100% for matched controls, P=0.03) and one-year postoperatively (50.8% vs. 83.3%, P=0.03) (17). The 30-day survival for replacement procedures in this trial was similar to previous, non-matched studies (12,24,25,33,34). The replacement cohort also experienced increased morbidity, although this could be due to the operative approach used—a difficult redo median sternotomy in a majority of cases, and a less invasive subcostal approach in five patients. Other case series have shown that this latter technique may suffice for some patients, with relatively low morbidity (25). Furthermore, limitations of this single controlled study included the inability to completely match groups for characteristics such as heart failure class and acuity of presentation (17). Clearly, further controlled studies investigating outcomes and costs associated with post-device exchange are needed, as well as guidelines on the indications and timing for replacement procedures.
This present review has several limitations. Firstly, all included studies were observational and retrospective. Thus, decisions regarding the indications for LVADs, operative approach used, and patient management strategies in device exchange were largely center-specific and driven by different surgeons, introducing a degree of selection bias. This could account for some of the variability seen with device failure rates and outcomes. Another potential contributor could be variations in study definitions of certain postoperative complications, such as pump thrombosis, and the different CF-LVAD models used across studies. In addition, data on mortality, morbidity, costs and quality of life were insufficiently reported to be included in analyses. Finally, survival and device failure rates reported in this review should be interpreted with a small margin of error as these figures were interpolated from digitalized curves for several included studies.
Conclusions
Device failure and replacement is associated with increased healthcare costs, morbidity and mortality. The present review highlighted the importance of device durability in optimizing patient outcomes as the average duration of long-term LVAD support increases. It also emphasized the need to investigate major causes of failure, particularly pump thrombosis, the pathophysiology of which remains unclear. Furthermore, improvements to LVAD design will play a key role in reducing the risk of complications such as lead fracture and device infection. To date, there remains a lack of guidelines and large, controlled studies reporting on the etiology, morbidity and mortality of LVAD failure.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Moazami N, Hoercher KJ, Fukamachi K, et al. Mechanical circulatory support for heart failure: past, present and a look at the future. Expert Rev Med Devices 2013;10:55-71. [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [PubMed]
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 2009;54:312-21. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Lee J; National Clinical Trial Initiative Subcommittee. Long-term Mechanical Circulatory Support System reliability recommendation by the National Clinical Trial Initiative subcommittee. ASAIO J 2009;55:534-42. [PubMed]
- Holman WL, Naftel DC, Eckert CE, et al. Durability of left ventricular assist devices: Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 2006 to 2011. J Thorac Cardiovasc Surg 2013;146:437-41.e1.
- Schechter MA, Daneshmand MA, Patel CB, et al. Outcomes after implantable left ventricular assist device replacement procedures. ASAIO J 2014;60:44-8. [PubMed]
- Stulak JM, Cowger J, Haft JW, et al. Device exchange after primary left ventricular assist device implantation: indications and outcomes. Ann Thorac Surg 2013;95:1262-7; discussion 1267-8. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Frazier OH, Gemmato C, Myers TJ, et al. Initial clinical experience with the HeartMate II axial-flow left ventricular assist device. Tex Heart Inst J 2007;34:275-81. [PubMed]
- John R, Kamdar F, Eckman P, et al. Lessons learned from experience with over 100 consecutive HeartMate II left ventricular assist devices. Ann Thorac Surg 2011;92:1593-9; discussion 1599-600. [PubMed]
- Moazami N, Milano CA, John R, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg 2013;95:500-5. [PubMed]
- Moazami N, Steffen RJ, Naka Y, et al. Lessons learned from the first fully magnetically levitated centrifugal LVAD trial in the United States: the DuraHeart trial. Ann Thorac Surg 2014;98:541-7. [PubMed]
- Potapov EV, Stepanenko A, Kaufmann F, et al. Thrombosis and cable damage in the HeartWare pump: clinical decisions and surgical technique. ASAIO J 2013;59:37-40. [PubMed]
- Sabashnikov A, Mohite PN, Zych B, et al. Outcomes and predictors of early mortality after continuous-flow left ventricular assist device implantation as a bridge to transplantation. ASAIO J 2014;60:162-9. [PubMed]
- Saito S, Yamazaki K, Nishinaka T, et al. Post-approval study of a highly pulsed, low-shear-rate, continuous-flow, left ventricular assist device, EVAHEART: a Japanese multicenter study using J-MACS. J Heart Lung Transplant 2014;33:599-608. [PubMed]
- Schechter MA, Daneshmand MA, Patel CB, et al. Outcomes after implantable left ventricular assist device replacement procedures. ASAIO J 2014;60:44-8. [PubMed]
- Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2013;32:675-83. [PubMed]
- Whitson BA, Eckman P, Kamdar F, et al. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg 2014;97:2097-103. [PubMed]
- Yuan N, Arnaoutakis GJ, George TJ, et al. The spectrum of complications following left ventricular assist device placement. J Card Surg 2012;27:630-8. [PubMed]
- Holman WL, Naftel DC, Eckert CE, et al. Durability of left ventricular assist devices: Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 2006 to 2011. J Thorac Cardiovasc Surg 2013;146:437-41.e1.
- Moazami N, Milano CA, John R, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg 2013;95:500-5. [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [PubMed]
- Adamson RM, Dembitsky WP, Baradarian S, et al. HeartMate left ventricular assist system exchange: results and technical considerations. ASAIO J 2009;55:598-601. [PubMed]
- Gregoric ID, Bruckner BA, Jacob L, et al. Clinical experience with sternotomy versus subcostal approach for exchange of the HeartMate XVE to the HeartMate II ventricular assist device. Ann Thorac Surg 2008;85:1646-9. [PubMed]
- Blitz A. Pump thrombosis—A riddle wrapped in a mystery inside an enigma. Ann Cardiothorac Surg 2014;3:450-71.
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [PubMed]
- Wu L, Weng YG, Dong NG, et al. Outcomes of HeartWare Ventricular Assist System support in 141 patients: a single-centre experience. Eur J Cardiothorac Surg 2013;44:139-45. [PubMed]
- Potapov EV, Kaufmann F, Stepanenko A, et al. Pump exchange for cable damage in patients supported with HeartMate II left ventricular assist device. ASAIO J 2012;58:578-82. [PubMed]
- Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant 2011;30:375-84. [PubMed]
- Koval CE, Thuita L, Moazami N, et al. Evolution and impact of drive-line infection in a large cohort of continuous-flow ventricular assist device recipients. J Heart Lung Transplant 2014. [Epub ahead of print]. [PubMed]
- Baronetto A, Centofanti P, Attisani M, et al. VAD infections: the lead, the graft and the pump. Ann Cardiothorac Surg 2014;3:505-6.
- Dembitsky WP, Tector AJ, Park S, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann Thorac Surg 2004;78:2123-9; discussion 2129-30. [PubMed]
- Stulak JM, Cowger J, Haft JW, et al. Device exchange after primary left ventricular assist device implantation: indications and outcomes. Ann Thorac Surg 2013;95:1262-7; discussion 1267-8. [PubMed]





