Clinical outcomes of hybrid repair for thoracoabdominal aortic aneurysms
Background: Thoracoabdominal aortic aneurysm (TAAA) hybrid repair consists of aortic visceral branch rerouting followed by TAAA endograft exclusion. This technique has been shown to represent a technically feasible strategy in selected patients.
Methods: We analyzed 52 high-risk patients who underwent hybrid TAAA repair between 2001 and 2012 in our centre with a variety of visceral rerouting configurations and of commercially available thoracic endografts. Thirty-seven simultaneous (71.2%) and 15 staged procedures (21.8%) were performed with a four-vessel revascularization in 18 cases (34.6%), a three-vessel revascularization in 11 cases (21.2%) and a two-vessel revascularization in 23 cases (44.2%).
Results: No intraoperative deaths were observed. We recorded a perioperative mortality rate of 13.5% (n=7), including deaths from multiorgan failure (n=2), myocardial infarction (n=2), coagulopathy (n=1), pancreatitis (n=1) and bowel infarction (n=1). Perioperative morbidity rate was 28.8% (n=15), including 2 cases of transient paraparesis and 1 case of permanent paraplegia. Renal failure (n=5), pancreatitis (n=3), respiratory failure (n=3) and dysphagia (n=1) were also observed. At median follow-up of 23.9 months procedure-related mortality rate was 9.6%: two patients died from visceral graft occlusion and three from aortic rupture. There were three endoleaks and one endograft migration, none of which resulted in death. Five patients (9.6%) died as a consequence of unrelated events.
Conclusions: Typical complications of conventional TAAA open surgery have not been eliminated by hybrid repair, and significant mortality and morbidity rates have been recorded. Fate of visceral bypasses and incidence of endoleak and other endograft-related complications needs to be carefully assessed. Hybrid TAAA repair should currently be limited to high-risk surgical patients with unfit anatomy for endovascular repair.
Key words: Thoracoabdominal; aorta; aneurysm; hybrid; endovascular
Introduction
The conventional open surgical treatment of TAAA over the past decades has been the inclusion technique as proposed by Crawford in 1978 (1). This challenging procedure has substantially evolved over the years particularly in organ protection strategies, enabling experienced surgical centres to have much lower mortality and morbidity rates than previously reported (2-9).
Furthermore, evolving technology has led to the extended use of endovascular grafts in the visceral aorta. In this critical position the experience with endografts incorporating the visceral vessels with either fenestrations or formal branches has been limited to few highly specialized centres conducting investigational studies (10-15). Few large series have been published and most of these reports suffer from a lack of accurate comparison with similar open procedures. Broader applicability and reports are available for hybrid TAAA repairs providing inflow to visceral arteries by means of extra-anatomic bypass followed by aortic endograft relining (16-25). Early favourable outcomes have encouraged some groups to also perform hybrid TAAA repair in good surgical patients (16).
In 2011 we published the outcomes of our series of 47 patients who underwent TAAA hybrid repair in our centre (25). In this paper we report the updated mid-term results of our single centre series consisting of 52 cases.
Methods
Indications and operative technique
From 1998 until 2012, 467 TAAA repairs were performed at our centre. From 2001 we identified 52 high-risk patients (39 males, median age 71.3 years, range, 23 to 83 years) who underwent TAAA hybrid repair by means of visceral/renal aortic debranching and endovascular exclusion of the aneurysm (20 type I, 6 type II, 10 type III, 6 type IV Crawford classification and 10 aneurysms of the visceral aortic patch). Inclusion criteria for hybrid TAAA repair included high-risk patients defined by American Society of Anaesthesiologists (ASA) class 3 or 4 associated with Forced Expiratory Volume in 1 Second <50% or cardiac ejection fraction <40%. One patient who did not fulfill these clinical criteria was selected for hybrid TAAA repair due to technical concerns related to a retroperitoneal fibrosis. Forty (76.9%) of these patients had a history of previous aortic surgery. Patient data was prospectively collected in a computerized database. The mean maximal TAAA diameter on the short axis was 69 mm (range, 64-91 mm). One patient underwent emergency treatment due to TAAA rupture. Anatomic inclusion criteria for hybrid repair was a minimum proximal aortic neck length of 20 mm, the probability of aortic visceral debranching to be effective in lengthening the distal aortic landing zone to greater than 15 mm, and an aortic neck diameter allowing 15% to 20% endograft oversizing with the absence of circumferential thrombus or calcifications.
In all cases, 16/64-row multi-detector spiral computed tomography (CT) with 1-mm intervals and multi-planar reconstruction was performed for planning and sizing. Thirty-seven simultaneous (71.2%) and 15 staged procedures (28.8%) were performed. Preoperative cerebro-spinal fluid drainage (CSFD) was instituted in 25 selected patients considered at high risk for spinal cord ischemia, this included: planned long segment coverage of native descending aorta by the endograft, and/or planned coverage of the intercostal artery that feeds the artery of Adamkiewicz, or in the case of prior AAA repair. The CSFD was instituted emergently in 2 patients who developed delayed postoperative symptoms of spinal cord ischemia. All patients received general anaesthesia and epidural analgesia.
A portable digital C-arm image intensifier with road-mapping capabilities (series 9600, OEC Medical System or Moonray, Simad Medical Technology, Modena, Italy) was used in all cases for the endovascular stage.
Visceral rerouting
Re-routing of the visceral aortic branches was performed by means of 148 retrograde bypasses through a midline laparotomy and transperitoneal access. Three antegrade visceral bypasses from the ascending aorta were performed through a median sternotomy associated with a median laparotomy. When the time of renal ischemia was longer than 10 minutes due to technical issues, such as cases requiring renal artery endarterectomy or a difficult anastomosis, renal perfusion was used. During renal ischemia, a bolus of 4 ℃ 300 mL of lactated Ringer's solution supplemented with mannitol 18% (70 mL) and 6-methylprednisolone (500 mg) or, since 2009, Custodiol solution, was perfused into the orifice of the renal artery with an occlusion perfusion balloon catheter (Pruitt- Inhara 9 Fr). A two-vessel revascularization was performed in 23 cases (44.2%), a three-vessel revascularization in 11 cases (21.6%) and a four-vessel revascularization in 18 cases (34.6%). Overall 151 visceral bypasses were performed (48 to the celiac axis, 51 to the superior mesenteric artery and 52 to the renal arteries). Diameters of visceral bypasses were 6-8 mm (146 Dacron and 5 expanded-polytetrafluoroethylene grafts). Customized Y-graft and single bypass were the preferred configuration (Figure 1). Reversed bifurcated or trifurcated grafts and single bypasses with sequential graft technique were also used.

For celiac trunk revascularization, we initially performed retropancreatic graft routing; currently the antepancreatic position is our preferred choice with an arteriotomy in the common hepatic artery and an end-to-side anastomosis. In case of anastomosis directly to the celiac trunk an end-to-end anastomosis is usually preferred.
An end-to-end anastomosis was usually preferred for the superior mesenteric arteries and for the renal arteries. In all reconstructions, the grafted vessels were ligated proximally to prevent type II endoleak. The grafts were then covered with retroperitoneum or omental flap whenever possible.
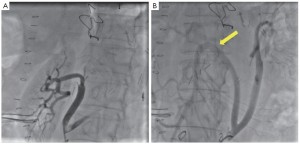
Completion angiography was always performed following debranching, as well as during staged procedures to assess the patency of visceral bypasses and to treat technical defects (Figure 2).
Inflow site
The choice of inflow site for retrograde visceral artery bypass grafting was based on the extent of the TAAA, the presence of prior abdominal aortic repair, and the quality of the walls of the native aorta and iliac arteries.
Patients with native aorto-iliac vessels (28 cases)
Among the 28 patients without prior abdominal aortic grafting, the native aorta represented the donor vessel in 6 cases (Figure 1A), while the common iliac artery was used as the inflow vessel in 16 cases. In 6 other patients, the infra-renal aorta was replaced for synchronous AAA during hybrid TAAA repair, with a tube graft in 4 patients and an aorto-bi-iliac bypass in 2 patients. The visceral bypasses were pre-sewn (Figure 1B) or anastomosed (Figure 1C) to the distal part of the tube grafts or to the distal part of the main body or to the iliac branches in patients with an aorto-bi-iliac reconstruction and were then routed to the target vessels.
Patients with pre-existing abdominal aortic graft (24 cases)
In 18 out of 24 cases with pre-existing abdominal aortic grafts for prior AAA repair or TAAA repair the retrograde grafts were anastomosed to the previous aortic graft. In 5 cases a pre-existing abdominal aortic graft was not used as the inflow site because of the good quality and favourable anatomic features of the common iliac arteries used as the donor vessels. In the last case with pre-existing abdominal aortic graft, due to a retroperitoneal fibrosis, 3 antegrade bypasses from the ascending aorta were performed.
Access site for endograft insertion
The access vessel for endograft insertion was the common femoral artery (exposed through an inguinal incision) in 34 patients, an iliac approach was used in 6 patients, and the device was inserted through the infrarenal aorta or an aortic graft in 9 patients. In these patients the access sites were repaired by purse-string suture, continuous direct suture or by a synthetic patch according to the size and the quality of the access site. A prosthetic conduit attached or pre-sewn (Figure 3) to aortic vascular graft or attached to native aorta was used in 4 cases of staged procedure, routed to the groin and then used for endograft deployment.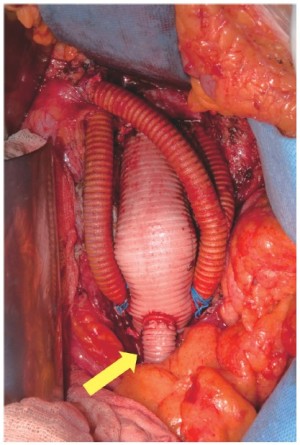
Aneurysm exclusion
Thoracoabdominal endovascular exclusion was achieved by means of different commercially available CE marked thoracic endografts and a mean of 1.5 stent grafts (range, 1 to 3) were deployed in each patient.
Completion angiography was always performed following deployment of the endografts both to assess the effective aneurysm exclusion and to check/confirm good patency of visceral bypasses.
Follow upPatients were evaluated with post-procedure contrast CT scans at scheduled follow-up intervals of 1, 6, and 12 months, and yearly thereafter (Figures 4,5). Clinical follow-up was also performed at regular 6-month intervals.
Results
Perioperative results
No intraoperative deaths were observed. Peri-operative mortality was 13.5% (7 patients). These included multiple organ failure in two, myocardial infarction in two, coagulopathy in one, pancreatitis in one and bowel infarction in one.
Overall major peri-operative morbidity was observed in 15 (28.8%) patients. Three cases of pancreatitis (8.8%) were observed, with associated abdominal fluid collection requiring percutaneous drainage at another centre in one patient. Five cases of peri-operative renal failure (9.6%) resolved without dialysis, and 3 cases of respiratory failure (5.8%) were also recorded. Delayed transient paraparesis was observed in 2 patients (3.8%) on postoperative day 2; both conditions resolved after cerebrospinal fluid drainage. One case (1.9%) of irreversible paraplegia was observed. One patient developed dysphagia and regurgitation after the endovascular procedure; esophageal imaging showed a marked endoluminal stenosis, suggesting the development of a secondary achalasia. The patient was submitted to botulinum toxin endoscopic injections at the lower esophageal sphincter, obtaining a complete resolution of symptoms (26).
Visceral arteries management
Overall, out of 155 planned visceral bypasses (148 retrograde, 3 antegrade from ascending aorta), only four target vessel revascularization (left renal artery in three and celiac trunk in one) failed due to technical issues.
One patient undergoing long-term dialysis had intentional coverage of both renal arteries, and 5 patients with a solitary functioning kidney had intentional coverage of the contralateral renal artery without revascularization. One patient had an aberrant common hepatic artery originating from the superior mesenteric artery, with a small anomalous celiac trunk, which was intentionally covered without revascularization. One patient had a gastro-hepatic trunk and the splenic artery originating independently from the aorta; the splenic artery was intentionally covered. In 1 patient with previous left nephrectomy and celiac trunk occlusion, only the right renal artery and the superior mesenteric artery were grafted. One patient with previous open type IV TAAA conventional repair by aortic grafting with bevelled proximal anastomosis developed severe retroperitoneal fibrosis and a type III TAAA; the left kidney was non-functional. Antegrade revascularization of celiac trunk, superior mesenteric artery and right renal artery was performed in this case by means of a trifurcated graft attached to the ascending aorta and routed through the diaphragm.
Patency of 18 (11.9%) visceral grafts in nine patients was intra-operatively assisted. One renal artery dissection, 12 renal artery stenoses, and 5 angulations with associated stenosis of the anastomosis of the superior mesenteric artery were detected during intra-operative angiography and immediately corrected with the deployment of a self-expanding stent (Figures 2,4,5). An initial dissection with mild stenosis of the right iliac artery representing the donor vessel for visceral rerouting was also stented intra-operatively distal to the proximal anastomosis of a “Y” graft because of a reduced femoral pulse following unclamping.
Mid-term results
At mean follow-up of 23.9±19 months, three patients died of sudden death potentially related to aortic rupture although autopsy was not performed in any case (one patient had a type II endoleak under follow up).
The rate of visceral graft occlusion was 7.3% (11/151) leading to bowel infarction and death in 2 patients and loss of a kidney in one patient. At six months post-procedure, a high grade stenosis of the superior mesenteric artery anastomosis leading to symptoms of abdominal angina and severe patient weight loss, was successfully treated with stenting.
One case of endograft migration leading to a type I endoleak required emergency complete visceral aortic debranching and endograft re-lining at another centre (we gratefully acknowledge Dr MP Jenkins-St Mary’s Regional Vascular Unit, Imperial College Healthcare NHS Trust, London, United Kingdom-for performing his successful operation).
Three type II endoleaks were detected: in one case from an aberrant left gastric artery originating directly from the aorta and in the other two from intercostal arteries. These leaks were not associated with aneurysmal enlargement.
One case of pancreatitis and one case of renal failure were also recorded. Seven non procedure-related deaths (2 myocardial infarction, 2 cancer, 1 cerebral aneurysm rupture, 1 head trauma and 1 unknown) were recorded.
Five patients were lost to follow-up.Discussion
Although open surgical repair of TAAA has evolved significantly over the last decades (2-9) , technical challenge and current morbidity and mortality of the inclusion technique are still significant, particularly in patients with extensive aneurysms or prior aortic surgery and in poor surgical candidates (12,21-27).
Currently, hybrid TAAA repair is an appealing technique and may represent a “bridge” solution as we wait for larger series and reproducible results from the evolving experience in total endovascular TAAA repair with fenestrated and branched endografts (10-14). By avoiding thoracotomy, we can hypothesize that hybrid TAAA repair may be particularly advantageous in patients with previous thoracic surgery. Especially in patients with “frozen-chest”, in which a redo left-sided thoracotomy may be associated with major bleeding and increased postoperative complications such as respiratory and organ failure (28-32). Hybrid repair may also have some advantages in cases of prior cardiac surgery and cannulation. In these cases pericardial or proximal aortic adhesions may increase the technical challenges and risk if an inclusion technique with distal perfusion through a left heart bypass is required. By avoiding thoracic aortic cross-clamping, hybrid TAAA repair should also be particularly appealing in patients with poor cardiac function and valvular heart disease.
However, based on a reported mortality rate of >30% in patients undergoing conventional open repair of extensive TAAA, some authors (16) encourage the use of hybrid TAAA repair rather than conventional surgery in good surgical patients. This may be a result of the usual poor clinical conditions of the patients, and a remarkable protective effect of hybrid treatment from typical complications of major surgery such as respiratory failure, coagulopathy and cardiac or renal complications. Furthermore we observed the occurrence of new threatening complications specific to hybrid procedures especially related to the visceral grafts such as bowel infarction, pancreatitis and renal artery thrombosis.
Spinal cord ischemia
It is still debated, whether the risk of paraplegia during endovascular thoracic aortic procedures is lower compared to open thoracic aortic surgery. During hybrid TAAA repair, the avoidance of supraceliac clamping and shortened duration of visceral ischemia, should lead to greater hemodynamic stability compared with conventional open repair, and thus in theory risk of spinal cord ischemia should be reduced (14). Böckler et al. demonstrated that in the animal model endovascular repair is associated with lower spinal cord ischemia and paraplegia rates than aortic cross-clamping (33). These findings were confirmed in humans by Carroccio et al. (34). This hypothetical advantage was likely balanced in our series by the increased risk of spinal cord ischemia related to the high incidence of prior descending, abdominal, or thoracoabdominal aortic graft repairs in the patients we selected for hybrid TAAA repair (24). However, the problem of paraplegia has not been eliminated by hybrid TAAA repair and extensive coverage of the thoraco-abdominal aorta could be identified as the cause of a significant rate of spinal cord complications.
Greenberg et al. (35) compared total endograft length in patients that did and did not develop neurological deficit, demonstrating a significant association with the length of aortic coverage. Spinal cord ischemia following thoracic endograft repair may be hypothesized to have a different aetiology with a better prognosis compared to open surgery (36), however only a borderline difference in spinal cord ischemia was found between the two techniques in the treatment of descending thoracic and thoracoabdominal aneurysm repair (37). We observed in our case series of hybrid TAAA repair delayed onset paraplegia with complete resolution after cerebrospinal fluid drainage - apattern quite unusual in our experience with open surgery.
Staged or simultaneous procedure
The choice of staged or simultaneous procedure is another debated issue of hybrid TAAA repair and it is related to the specific clinical conditions of each individual case.
The staged strategy reduces the burden of procedure and theoretically reduces the risk of respiratory failure related to a prolonged tracheal intubation and the risk of coagulopathy related to aneurysm thrombosis associated with hypothermia and with the extensive surgical procedure required for visceral artery rerouting.
Regarding the risk of spinal cord ischemia, the role of staged repair as preventive strategy is still debated. Controlled temporary perfusion of the aneurysm sac and its segmental vessels via perfusion branches arising from the graft has been proposed as an adjunct to provide spinal cord perfusion during the immediate postoperative period (when the risk of spinal cord ischemia is greatest) after totally endovascular TAAA repair (38); the aneurysm exclusion is completed by occluding the perfusion branches 7 to 10 days after the first procedure. In the same way, a staged hybrid TAAA repair with delayed TEVAR, should have the potential to guarantee intercostal artery perfusion in the critical postoperative time after visceral debranching.
In cases of staged repair in patients with inadequate femoral and iliac arteries, prosthetic conduits pre-sewn or attached to the abdominal aorta can be routed to the groin to avoid possible access difficulties during the second stage of procedure (Figure 3). These side branches should be used selectively because they may increase the risk of infection and thrombotic embolization during thrombectomy of a thrombosed conduit before endograft insertion.
The simultaneous strategy has the advantages of eliminating the risk of interval TAAA rupture and offers an easy iliac or aortic access site when the femoral arteries are not adequate.
Ideal inflow siteRegarding the ideal inflow-site for visceral bypasses, a previous abdominal aortic graft may theoretically be the best inflow site for the visceral bypasses, avoiding clamping and suturing on atherosclerotic native arterial wall. However, during the endovascular stage an anterior take-off of visceral grafts from native or grafted aorta is not easy to identify during angiography with a portable “C-arm”. Thus, the common iliac artery inflow site may make the endograft positioning and deployment easier.
New quadrifurcated grafts with pre-sewn side branches designed for the revascularization of lower limbs and pelvic arteries (Hemagard knitted quadrifurcated) are currently commercially available and could play an interesting role in reno-visceral revascularization.
Visceral bypass outcomes
The long-term patency and safer routes of visceral bypass are other matters of concern, and some devastating complications such as visceral graft occlusion and pancreatitis must be carefully assessed. Currently, the ante-pancreatic route is preferred for the vascularisation of the hepatic artery, in order to reduce the risk of pancreatic injury via the posterior tunnel. Baseline angiography of the visceral bypasses is performed in both single and two-stage interventions with special care in checking for the absence of flow-limiting technical defects such as stenosis, visceral vessel dissection, or kinked anastomosis or angulated or twisted grafts after bowel repositioning. In these cases prompt correction with a self-expanding stent has been consistently safe and effective in our series (Figures 2,4,5). In one patient who experienced a fatal bowel infarction during the inter-surgical interval, no completion angiography was performed following visceral artery rerouting.
Regarding the long-term outcomes of visceral grafts, the risk of late restenosis needs to be carefully assessed and adjunctive endovascular procedures to assist patency are recommended in case of significant stenosis and angulation. Also the risk of enteric erosion or fistula of visceral grafts in their extra-anatomic route will need to be closely monitored.
Interestingly new sutureless telescoping anastomotic technique using GORE®Viabahn® self-expanding endograft (W.L. Gore & Associates, Inc, Flagstaff, AZ, USA) has been recently proposed to minimize vessel dissection and ischemia during renovisceral vascularisation in hybrid TAAA repair (39).
Also techniques of partial visceral debranching combined with the use of covered periscope and chimney grafts that have been shown to be safe and feasible with satisfactory patency and low incidence of endoleak (40,41) may be an alternative option to treat complex pararenal and thoracoabdominal pathologies.
Conclusions
Based on our series of selected high-risk patients, many concerns such as the long-term durability of endograft materials, outcomes of visceral bypasses, and the fate of the excluded TAAA, still remain unsolved. Larger study cohorts with longer follow up are needed to make statistically meaningful comparisons with standard open surgery and account for all the biases related to the learning curve, continuous technical progress and materials improvements typical of the evolving nature of hybrid TAAA repair.
Currently, standard open surgery through the inclusion technique in high-volume centres is the gold standard for TAAA treatment (6). Hybrid repair should still be reserved as an alternative to observation in patients unfit for the inclusion technique (32).
Acknowledgements
We gratefully acknowledge the contribution by Dr. George Lee in the English revision of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Crawford ES, Snyder DM, Cho GC, et al. Progress in treatment of thoracoabdominal and abdominal aortic aneurysms involving celiac, superior mesenteric, and renal arteries. Ann Surg 1978;188:404-22.
- Coselli JS. The use of left heart bypass in the repair of thoracoabdominal aortic aneurysms: current techniques and results. Semin Thorac Cardiovasc Surg 2003;15:326-32.
- Coselli JS, Conklin LD, LeMaire SA. Thoracoabdominal aortic aneurysm repair: review and update of current strategies. Ann Thorac Surg 2002;74:S1881-4; discussion S1892-8.
- Schepens M, Dossche K, Morshuis W,et al. Introduction of adjuncts and their influence on changing results in 402 consecutive thoracoabdominal aortic aneurysm repairs. Eur J Cardiothorac Surg 2004;25:701-7.
- Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 1999;67:1931-4; discussion 1953-8.
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4; discussion S890-2.
- Engle J, Safi HJ, Miller CC 3rd, et al. The impact of diaphragm management on prolonged ventilator support after thoracoabdominal aortic repair. J Vasc Surg 1999;29:150-6.
- Cambria RP, Clouse WD, Davison JK, et al. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg 2002;236:471-9; discussion 479.
- Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9.
- Greenberg RK, Lytle B. Endovascular repair of thoracoabdominal aneurysms. Circulation 2008;117:2288-96.
- Chuter TA, Rapp JH, Hiramoto JS, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2008;47:6-16.
- Dardik A, Perler BA, Roseborough GS, et al. Aneurysmal expansion of the visceral patch after thoracoabdominal aortic replacement: an argument for limiting patch size? J Vasc Surg 2001;34:405-9; discussion 410.
- Chiesa R, Melissano G, Civilini E, et al. Two-stage combined endovascular and surgical approach for recurrent thoracoabdominal aortic aneurysm. J Endovasc Ther 2004;11:330-3.
- Chiesa R, Melissano G, Marrocco-Trischitta MM, et al. Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J Vasc Surg 2005;42:11-7.
- Guillou M, Bianchini A, Sobocinski J, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2012;56:65-73.
- Black SA, Wolfe JH, Clark M, et al. Complex thoracoabdominal aortic aneurysms: endovascular exclusion with visceral revascularization. J Vasc Surg 2006;43:1081-9; discussion 1089.
- Böckler D, Kotelis D, Geisbüsch P, et al. Hybrid procedures for thoracoabdominal aortic aneurysms and chronic aortic dissections - a single center experience in 28 patients. J Vasc Surg 2008;47:724-32.
- Resch TA, Greenberg RK, Lyden SP, et al. Combined staged procedures for the treatment of thoracoabdominal aneurysms. J Endovasc Ther 2006;13:481-9.
- Lee WA, Brown MP, Martin TD, et al. Early results after staged hybrid repair of thoracoabdominal aortic aneurysms. J Am Coll Surg 2007;205:420-31.
- Zhou W, Reardon M, Peden EK, et al. Hybrid approach to complex thoracic aortic aneurysms in high-risk patients: surgical challenges and clinical outcomes. J Vasc Surg 2006;44:688-93.
- Chiesa R, Tshomba Y, Melissano G, et al. Hybrid approach to thoracoabdominal aortic aneurysms in patients with prior aortic surgery. J Vasc Surg 2007;45:1128-35.
- Tshomba Y, Bertoglio L, Marone EM, et al. Visceral aortic patch aneurysm after thoracoabdominal aortic repair: conventional vs hybrid treatment. J Vasc Surg 2008;48:1083-91.
- Chiesa R, Tshomba Y, Melissano G, et al. Is hybrid procedure the best treatment option for thoraco-abdominal aortic aneurysm? Eur J Vasc Endovasc Surg 2009;38:26-34.
- Chiesa R, Tshomba Y, Marone EM, et al. Hybrid procedures for the treatment of thoracoabdominal aortic aneurysms and dissections. J Cardiovasc Surg (Torino) 2010;51:821-32.
- Chiesa R, Tshomba Y, Logaldo D, et al. Hybrid repair of aortic aneurysms and dissections: the European perspective. Tex Heart Inst J 2011;38:687-90.
- Kahlberg A, Marrocco-Trischitta MM, Marone EM, et al. An unusual case of dysphagia after endovascular exclusion of thoracoabdominal aortic aneurysm. J Endovasc Ther 2009;16:238-42.
- Tshomba Y, Melissano G, Civilini E, et al. Fate of the visceral aortic patch after thoracoabdominal aortic repair. Eur J Vasc Endovasc Surg 2005;29:383-9.
- Nawa Y, Masuda Y, Imaizumi H, et al. Comparison of surgical versus endovascular stent-graft repair of thoracic and thoracoabdominal aortic aneurysms in terms of postoperative organ failure. Masui 2004;53:1253-8.
- Kawaharada N, Morishita K, Fukada J, et al. Thoracoabdominal aortic aneurysm repair through redo left-sided thoracotomy. Ann Thorac Surg 2004;77:1304-8.
- Kawaharada N, Morishita K, Fukada J, et al. Surgical treatment of thoracoabdominal aortic aneurysm after repairs of descending thoracic or infrarenal abdominal aortic aneurysm. Eur J Cardiothorac Surg 2001;20:520-6.
- Menard MT, Nguyen LL, Chan RK, et al. Thoracovisceral segment aneurysm repair after previous infrarenal abdominal aortic aneurysm surgery. J Vasc Surg 2004;39:1163-70.
- Baril DT, Carroccio A, Ellozy SH, et al. Endovascular thoracic aortic repair and previous or concomitant abdominal aortic repair: is the increased risk of spinal cord ischemia real? Ann Vasc Surg 2006;20:188-94.
- Böckler D, Kotelis D, Kohlhof P, et al. Spinal cord ischemia after endovascular repair of the descending thoracic aorta in a sheep model. Eur J Vasc Endovasc Surg 2007;34:461-9.
- Carroccio A, Marin ML, Ellozy S, et al. Pathophysiology of paraplegia following endovascular thoracic aortic aneurysm repair. J Card Surg 2003;18:359-66.
- Greenberg R, Resch T, Nyman U, et al. Endovascular repair of descending thoracic aortic aneurysms: an early experience with intermediate-term follow-up. J Vasc Surg 2000;31:147-56.
- Chiesa R, Melissano G, Bertoglio L, et al. The risk of spinal cord ischemia during thoracic aorta endografting. Acta Chir Belg 2008;108:492-502.
- Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation 2008;118:808-17.
- Harrison SC, Agu O, Harris PL, et al. Elective sac perfusion to reduce the risk of neurologic events following endovascular repair of thoracoabdominal aneurysms. J Vasc Surg 2012;55:1202-5.
- Rancic Z, Mayer D, Pfammatter T, et al. A new sutureless telescoping anastomotic technique for major aortic branch revascularization with minimal dissection and ischemia. Ann Surg 2010;252:884-9.
- Donas KP, Pecoraro F, Torsello G, et al. Use of covered chimney stents for pararenal aortic pathologies is safe and feasible with excellent patency and low incidence of endoleaks. J Vasc Surg 2012;55:659-65.
- Pecoraro F, Pfammatter T, Mayer D, et al. Multiple periscope and chimney grafts to treat ruptured thoracoabdominal and pararenal aortic aneurysms. J Endovasc Ther 2011;18:642-9.





