Endovascular repair of thoracoabdominal aneurysms: results of the first 48 cases
Background: In 2006, we began our experience with a novel technology for fully endovascular thoracoabdominal aneurysm repair, based on a custom-made, branched stent graft design. After 48 cases, we have learned and achieved substantial progress both in technical and in clinical skills. This paper describes the partial results of this ongoing experience.
Methods: Patients in this series were selected for the presence of thoracoabdominal aortic aneurysms, with or without dissection, which was present in one patient. The observation of extensive anatomical variations in several patients prompted changes in many of the basic stent graft configurations, which are also described.
Results: Between August 2006 and June 2012, 48 patients were treated consecutively with custom-made branch stent grafts. The five patients with the longest follow-up available so far are at 71, 65, 60, 54 and 51 months post-procedure. The operative mortality rate, defined as death during or within a month of surgical hospitalization, was 21% (10 patients); each case is described herein. During postoperative follow up, nine patients died from causes not directly related to aneurysmal disease, at 3, 18, 20, 22, 24, 24, 37, 44 and 46 months. The main causes of death were myocardial infarction (four cases), cancer (two cases), gastrointestinal hemorrhage (one case), ischemic stroke (one case), and sepsis (one case). Permanent paraplegia occurred in one patient.
Conclusions: It is still too soon to compare the results of endovascular repair of thoracoabdominal aneurysms with those of open surgical series. Despite the active and rapid progress currently observed for the endovascular method, it is still far from reaching its state-of-the-art plateau or becoming a gold standard. Further technological and technical advances in endovascular stent grafting seem to have a clear potential to provide very satisfactory operative outcomes for thoracoabdominal aortic aneurysms.
Key words: Aortic aneurysm; thoracoabdominal; endovascular procedures; stents
IntroductionOther Section
In 1956, Dr. Michael DeBakey warned of the “grave significance” of thoracoabdominal aortic aneurysms (TAAA) with major visceral branch involvement (1). More than 50 years later, major progress in technical and clinical skills has allowed many centers of excellence to achieve outstanding morbidity and mortality outcomes (2-4). However, for most centers worldwide, these achievements still seem far from reality, and the grave significance of TAAA still holds true (5,6) In parallel, development of endovascular surgery has progressed sharply, to the point where it may overtake open surgery as the main choice for treatment of thoracic and abdominal aortic aneurysms, as even centers with high volume and excellent results with open techniques are seeing a trend among surgeons to move towards the endovascular approach (7). In future, this could be the case for thoracoabdominal aortic aneurysms as well.
In late 2006, after a long learning curve in the endovascular management of abdominal aortic aneurysms, we began our experience with a novel stent graft technology based on a custom-made branch design (8). After 48 cases, we have learned and evolved in both technical and clinical skills, although much further development of endovascular surgery is still required before this technique can achieve the gold standard and compare with open thoracoabdominal outcomes.
MethodsOther Section
Patients with TAAAs and severe comorbidities, expected to be at high risk for a regular open procedure, were considered for endovascular repair, following current indications on the basis of aneurysm size (at least 5.5 cm in diameter) or rapid growth (0.5 cm during the 6 months preceding surgery). This series also included one patient with a combined aortic dissection and large TAAA. Once arterial anatomy was considered suitable for repair on the basis of computed tomography angiography (CTA) with 1 mm slice thickness, patients were asked to provide informed consent for the procedure. Chest, abdomen and pelvic CTA were assessed by the authors using adjunctive three-dimensional analysis with the OsiriX software (OsiriX Foundation, Geneva, Switzerland). Custom-made grafts were then designed and submitted to the planning team at Cook Australia, Inc. (Brisbane, Australia) for fine-tuning according to their own CTA analyses and device manufacturing procedures.
The study was approved by the Research Ethics Committee (institutional review board-equivalent) of the institution. All patients provided written informed consent for their participation in the study.
Device planning and design and operative strategies
The custom-made branched stent graft devices are constructed based on the Zenith® AAA stent graft platform (Cook Medical, Brisbane, Australia), which comprises stainless steel Z-stents and woven polyester fabric of the same thickness of regular open surgery grafts. The key difference is the presence of a reducing stent and side branches (SBs). The reducing stent, which is located distal to the sealing zone, converts the main body into a 16-, 18- or 22-mm cuff-bearing segment. This diameter is planned on the basis of aortic lumen size, so that it can accommodate both the main body and the bridging covered stents between the graft SBs and the visceral vessels. The diameter of the distal segment of the graft is adjusted to be sealed either to the diameter of the previous graft (in patients who have already undergone open or endovascular infrarenal aneurysm repair) or grow back to a diameter of 22 mm, which will serve as a standard diameter for the overlap of a distal 24-mm bifurcated stent graft, which is placed simultaneously.
The most common stent graft set was composed of a four-side-branch stent graft and a bifurcated stent graft with iliac extensions, similar to regular aortic stent grafts except for the absence of a free-flow stent, which is not recommended when the graft is to be overlapped within the distal stent graft. Nonetheless, some modifications were tailored to each patient’s anatomy. These included:
• in patients with an extra renal artery or a hepatic artery arising directly from the aorta, we used a five-branched stent graft (Figure 1A);
• in patients with Crawford type I aneurysms, the graft may have a scallop at the distal stent, allowing flow to the renal arteries without the need for stenting. In our series, this was especially useful for one patient who had multiple renal arteries on the same side (Figure 1B);
• for patients with Crawford type I aneurysms, we also planned stent grafts with proximal side branches for the celiac and superior mesenteric arteries and fenestrations for the renal arteries, completed with covered balloon-expandable stents bridging the main stent graft and the renal arteries (Figure 1C);
• in patients whose renal arteries arose in a somewhat upward direction, making catheterization from the preferred access site (axillary) difficult, we used customized inverted side branches, which are catheterized through a femoral access (Figure 1D).
• to reduce the extension of healthy aorta required for the landing zone in patients with a type IV aneurysm, we used grafts with an inner side branch to the celiac trunk. This strategy also helps prevent spinal cord ischemia (Figure 1E);
• in patients expected to exhibit tortuous anatomy, we also employed a preloaded catheter and guidewire, which facilitate advancement of the sheath into the side branch, with the aid of an endovascular snare;
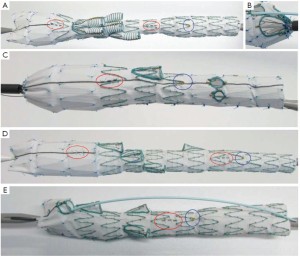
• in patients with associated iliac aneurysms, branch iliac devices were implanted before the aortic branched stent grafts;
• in patients with aneurysms at the level of the descending thoracic aorta, thoracic stent grafts (Zenith TX-2, Cook Medical) may be used proximally, creating a proximal landing zone for the branched stent graft.
The usual bridging stent system between side branch and visceral artery consisted of a self-expandable covered stent (Fluency, C. R. Bard, Murray Hill, NJ) followed by a self-expandable bare stent within it (Zilver, Cook Medical, Brisbane, Australia) to provide extra kink resistance.
To create a soft transition from the bridging stent into the visceral artery, the bare stent is usually deployed 5 mm to 10 mm further inside the renal arteries.
In two cases, while catheterizing the celiac trunk, we were surprised to find an artery no longer patent in comparison to the CT scan used for planning. This may have been due to the time elapsed between planning and graft delivery, unstable disease, or both. We prefer to use a rather recent CT scan (obtained three months prior to surgery at most), with 1 mm or thinner axial slices. In such cases, when necessary, the side branch is occluded by deploying the covered stent filled with coils or, preferably, with a vascular plug (9).
To reduce the risk of spinal cord ischemia, in addition to reducing the extent of healthy aorta coverage, we also place a spinal catheter in all patients. Cerebrospinal fluid (CSF) is drained to an external closed CSF drainage system, which is set to maintain pressure below 10 mmHg during the first 48 hours postoperatively, regardless of the presence or absence of neurologic symptoms. While at the ICU, the target mean blood pressure is 90 mmHg, based on strict monitoring of urine output and echocardiography to control volume replacement. Continuous small doses of norepinephrine are sometimes used to keep blood pressure at target level, but only when volume replacement is considered ideal.
Bilateral exposure of the common femoral arteries is achieved with a transverse incision 2 cm above the inguinal ligament, through which the branch and abdominal stent grafts are implanted. In 2012, we changed the site of axillary artery exposure from the axillary region to the first portion of the axillary artery, a location similar to that used for axillofemoral bypass. This exposure provides a larger-diameter segment of the artery and decreases the risk of brachial nerve injury in comparison with axillary exposure, as performed earlier.
At this point, before moving to the axillary access, the femoral arteries can be reconstructed to allow perfusion of the lower extremities, sparing the patient the metabolic consequences of ischemia.
To stabilize the sheath system, a 0.014"×300 mm wire is inserted via a radial puncture of the 12 Fr sheath valve, using a through-and-through technique from the axillary artery and exiting at a femoral artery suture line. A longer 9 Fr sheath, which will actually guide the bridging stent grafts into the visceral arteries, is then inserted.
To minimize iodinated contrast use, we only perform the first arteriogram after the branched stent graft is inside the aorta, as the configuration of the aorta and visceral arteries usually changes once the sheath and stent graft are in place. Usually, 20 mL of 50% iodixanol (Visipaque, GE Healthcare, Little Chalfont, UK) in saline solution at 900 psi pressure and 10 mL/s flow will provide enough guidance to open the stent graft. All secondary contrast injections, to localize the visceral arteries, confirm positioning, or rule out endoleak, are performed with a solution of 3 mL iodixanol in 7 mL saline, injected manually. Isosorbide mononitrate (10 mg) is injected into each visceral artery once the guidewire is in place, to prevent vascular spasm before stent insertion. Control arteriograms of the entire system are also obtained using the aforementioned 50% iodixanol solution.
Patients are kept on double antiplatelet therapy (clopidogrel 75 mg and acetylsalicylic acid 200 mg daily) for at least 3 months, followed by indefinite single-agent therapy with clopidogrel or acetylsalicylic acid thereafter. Control CTA is performed at 1, 6, and 12 months after surgery and yearly thereafter.
ResultsOther Section
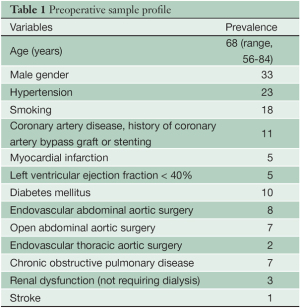
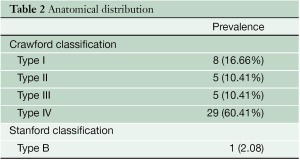
Between August 2006 and June 2012, 48 patients were treated consecutively with custom-made branched stent grafts. The main demographic characteristics of the series and anatomical distribution of aneurysms are shown in Tables 1 and Table 2 respectively.
Full table
Full table
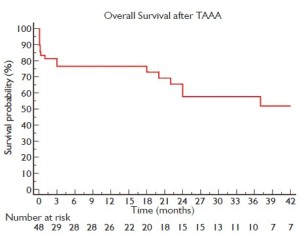
Figure 2 shows a Kaplan-Meier curve of overall survival of patients up to 42-month follow-up (limited to 10% standard error). The operative mortality rate, defined as death during surgery or during the whole surgery-related hospitalization period, was 21% (10 patients). All deaths are described in greater detail in Table 3.
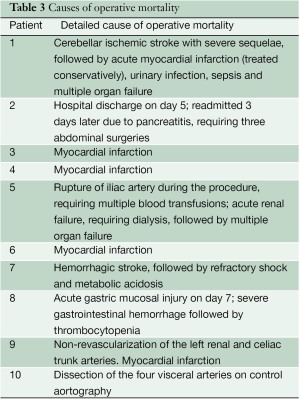
Full table
During the postoperative follow-up period, nine patients died from causes not directly related to aneurysmal disease at 3, 18, 20, 22, 24, 24, 37, 44 and 46 months. The leading causes of death were myocardial infarction (four cases), cancer (two cases), gastrointestinal hemorrhage (one case), ischemic stroke (one case), and sepsis (one case).
Operative morbidity is described in Table 4, according to frequency of complications. All 48 patients were extubated after surgery and could be assessed for paraplegia.
Paraplegia occurred in two patients, but resolved completely in one after intensification of CSF drainage and elevation of mean arterial blood pressure, followed by physical therapy. Renal failure requiring dialysis in the postoperative period was directly related to mortality, and none of the patients who developed this complication survived, although it was not an isolated event in any of the cases. Myocardial infarction was the leading cause of operative mortality, accounting for four cases.
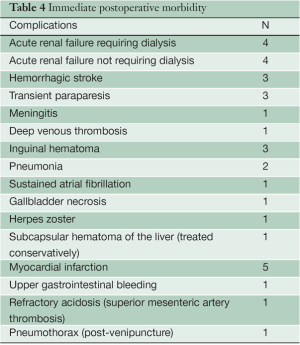
Full table
Technical success, defined as implantation of the entire stent graft set and bridging stents to the patent visceral arteries, was achieved in all but two cases. In one patient, all four visceral arteries dissected during implantation of the bridging stents and attempts at revascularization failed, causing death. The other patient, who had a history of stent graft placement for abdominal aortic aneurysm repair, had a free-flow stent in front of the visceral arteries that did not allow proper catheterization of the left renal and celiac trunk. This patient suffered a myocardial infarction and died 24 hours later.
Contrast volume and operative time varied widely. Mean contrast volume was 160 mL (range, 48 to 295 mL) and mean operative time was 8.3 hours (range, 3 to 15 hours). The mean length of hospital stay was 10.2 days (range, 4 to 120 days).The five patients with the longest follow-up available thus far are at 71, 65, 60, 54 and 51 months postprocedure respectively. In this group, only one required secondary surgery, 18 months after graft placement, to correct disconnection of a previous iliac limb caused by the passage of the 24 Fr sheath of the branched stent graft that went unnoticed during the procedure. Together, these five patients account for a total of 19 visceral arteries revascularized without complications.
Out of a total of 180 successfully revascularized visceral arteries, two reinterventions were required. In one patient, we observed partial disconnection of the celiac trunk, in which the bare stent was still within the artery but the covered stent migrated into the aneurysmal sac, leading to a large endoleak. This was corrected with placement of a second covered stent. In another patient with stable renal impairment not requiring dialysis, chronic thrombosis of the left renal branch was detected at duplex scan 12 months after graft placement, when the patient stopped antiplatelet therapy. Aspiration thrombectomy of the affected branch was performed successfully with the AngioJet system (Medrad Inc., Warrendale, PA), but there was no decrease in creatinine levels. We did not observe stenosis indicative of hyperplasia in any control study cases, including those with longer follow-up.
DiscussionOther Section
Our review of this initial experience in the treatment of with a fully endovascular technique takes us back to the words of the vascular surgery pioneers who first attempted to treat patients with this disease, as TAAAs still carry “considerable danger of producing fatal ischemic damage to such vital structures as the liver, kidneys, and gastrointestinal tract” (1). We would add to this list the heart and spinal cord, which are affected by the severe inflammatory state and direct ischemia caused by TAAAs, respectively–still challenges to overcome. Whereas endovascular techniques for infrarenal abdominal aortic aneurysm repair seem to have reached a high degree of effectiveness, TAAA repair still seems to be on an ascending curve towards better outcomes.
This was certainly the case in our learning curve of these first 48 cases. We had to develop a wide range of technical and clinical skills to overcome the anatomical and clinical challenges associated with planning, design and implantation of the custom-made stent grafts used in our series and to manage postoperative outcomes.
Some of the complications seen with the endovascular technique are similar to those observed with open repair, such as spinal cord ischemia; so far, we have employed some strategies derived from management of this complication in the open technique-namely, drainage of cerebrospinal fluid and 0-degree elevation during the first 48 hours post-procedure. We also try to save the internal iliac arteries, which provide collateral arterial flow to the spinal cord, whenever possible. So far, there have only been one case of permanent paraplegia, one case of transient paraplegia (which resolved completely within three months of surgery) and two cases of transient paraparesis in our series. As endovascular techniques are quickly evolving, innovative approaches to prevention of this dreaded complication are constantly being proposed, and some of these may prove effective (10,11).
The observation of anatomical variations and recognition of the attendant potential for difficulties in catheterization has led us to implement several custom-made changes to the basic four-branch stent graft configuration. For instance, some renal arteries take off upward from their origin at the aorta, or are located far from the central aortic lumen; in these cases, we now tend to use inverted branches, which are catheterized via the femoral rather than axillary route. As mentioned, we also planned stent grafts with only one scallop on the distal stent to accommodate two renal arteries not involved by the aneurysm but located at the distal landing zone of the stent graft. This has allowed us to preserve renal function, as the renal arteries were not excluded.
We have learned that, to reduce metabolic complications, the femoral artery access should be reconstructed as soon as possible. Therefore, once the set of stent grafts are in place, we perform balloon accommodation (sealing of the graft by means of balloon inflation) and place arterial sutures. On one side, the 0.014" guidewire used to stabilize the sheath system inserted through the axillary access exits the suture line and is removed gently after placement of all bridging stents.
The first half of our cases was performed with C-arm X-ray equipment, causing long operative times and low image quality throughout the procedure, which we find unacceptable in the endovascular suite era, although our center still lacks this technology for socioeconomic reasons. Later procedures were, and have been, performed using cath lab equipment (Axiom Artis, Siemens, Munich, Germany), which has drastically improved operative times and contrast volumes.
However, our overall mortality rate is still not satisfactory, and is much higher than that reported by other centers using the same technology (12-14). We are, of course, committed to searching for-and addressing-the reasons behind this. One such reason was the adverse clinical profile of our group of patients, with a high incidence of severe coronary disease, which was probably underestimated during preoperative assessment as it was the isolated main cause of death in four cases.
Despite that, we have also observed the “onoff” phenomenon described by Guillou et al. (13), in which patients who did not develop any in-hospital complications during the first postoperative days had a very uneventful follow-up (considering the 26 patients in our series with >12 months follow-up). All aneurysmor procedure-related deaths occurred during the operative period. This might have important implications in future, once proper patient selection criteria have been established and we are able to identify which patients should not be offered surgery at all.
Our overall opinion of endovascular repair is that it is still too soon to compare its results with those of open surgical series, as the endovascular method is, despite active and rapid progress, still far from reaching its state-of-the-art plateau or “gold standard” status, which the open technique seems to have achieved in centers of excellence.
We believe that further technological and technical advances in endovascular stent grafting have a clear potential to provide very satisfactory operative outcomes for this deadly disease.
AcknowledgementsOther Section
Disclosure: Marcelo Ferreira is a proctor for Cook Medical in Latin America. The other authors declare no conflict of interest.
ReferencesOther Section
- DeBakey ME, Creech O Jr, Morris GC Jr. Aneurysm of thoracoabdominal aorta involving the celiac, superior mesenteric, and renal arteries; report of four cases treated by resection and homograft replacement. Ann Surg 1956;144:549-73.
- Bakaeen FG, Chu D, Huh J, et al. Contemporary outcomes of open thoracic aortic surgery in a veteran population: do risk models exaggerate mortality? Am J Surg 2009;198:889-94.
- Coselli JS, LeMaire SA. Tips for successful outcomes for descending thoracic and thoracoabdominal aortic aneurysm procedures. Semin Vasc Surg 2008;21:13-20.
- Jacobs MJ, Mommertz G, Koeppel TA, et al. Surgical repair of thoracoabdominal aortic aneurysms. J Cardiovasc Surg (Torino) 2007;48:49-58.
- Cowan JA Jr, Dimick JB, Henke PK, et al. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg 2003;37:1169-74.
- Rigberg DA, McGory ML, Zingmond DS, et al. Thirtyday mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg 2006;43:217-22; discussion 223.
- Conrad MF, Crawford RS, Pedraza JD, et al. Long-term durability of open abdominal aortic aneurysm repair. J Vasc Surg 2007;46:669-75.
- Ferreira M, Lanziotti L, Monteiro M. Branched devices for thoracoabdominal aneurysm repair: early experience. J Vasc Surg 2008;48:30S-36S; discussion 36S.
- Ferreira M, Monteiro M, Lanziotti L. How to occlude a side branch on a branched stent-graft during an endovascular thoracoabdominal aortic aneurysm repair. J Endovasc Ther 2009;16:454-6.
- Harrison SC, Agu O, Harris PL, et al. Elective sac perfusion to reduce the risk of neurologic events following endovascular repair of thoracoabdominal aneurysms. J Vasc Surg 2012;55:1202-5.
- Lioupis C, Corriveau MM, Mackenzie KS, et al. Paraplegia prevention branches: a new adjunct for preventing or treating spinal cord injury after endovascular repair of thoracoabdominal aneurysms. J Vasc Surg 2011;54:252-7.
- Clough RE, Modarai B, Bell RE, et al. Total endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2012;43:262-7.
- Guillou M, Bianchini A, Sobocinski J, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2012;56:65-73.
- Reilly LM, Rapp JH, Grenon SM, et al. Efficacy and durability of endovascular thoracoabdominal aortic aneurysm repair using the caudally directed cuff technique. J Vasc Surg 2012;56:53-64.