Deep versus mild hypothermia during thoracoabdominal aortic surgery
Introduction
In 1950, Bigelow et al. (1) demonstrated that hypothermic canines tolerated circulatory exclusion of the heart for longer periods of time than normothermic canines. Shortly thereafter, F. John Lewis applied the ideas of hypothermia clinically by closing an atrial septal defect in a pediatric patient (2). In 1958, Sealy et al. (3) successfully combined hypothermia with cardiopulmonary bypass (CPB) in a wide range of patients undergoing cardiac surgery. Deep hypothermic circulatory arrest (DHCA) was first used in aortic surgery in 1975 in a series of patients undergoing aortic arch replacements (4). Since this time, DHCA has been broadened in its applications to all types of aortic surgery including descending thoracic aortic aneurysms (DTA) and thoracoabdominal aortic aneurysms (TAAA). Launched by these early experiences, hypothermia has become the single most effective tissue preservation adjunct necessary during specific cardiac and aortic surgeries that require reduced or even no blood flow.
The rationale behind the use of hypothermia lies in its protective effect on organs via a reduction in metabolic rate and oxygen consumption, preservation of highenergy phosphate storages, and decreased central nervous system excitatory neurotransmitter release (5-7). One study investigating metabolic activity in experimental pigs found the cerebral metabolic rate to be 50% of baseline at 28 °C, 19% at 18°C, and 11% at 8 °C. Thus, the relationship between cerebral metabolic rate as a function of temperature was exponential with a Q10 (degree of metabolic suppression with a 10 °C drop in temperature) to be approximately 2.46 (8). These findings demonstrated an appreciable temperature-dependent decrease in metabolic activity even between moderate hypothermia (18 °C) and deep hypothermia (8 °C). Later, McCullough and colleagues (9) in an elegant study in humans estimated a similar Q10 of 2.3 by calculating differences in metabolic activity during mild, moderate, and deep hypothermic circulatory arrest. These studies were based off of the clinical observation that return to baseline brain function is possible after cessation of blood flow at normothermia for no longer than five minutes. After a number of studies demonstrated shortand long-term neurological and cognitive dysfunction with longer ischemic times (10-13), this eventually led to the determination that the maximum safe DHCA time was approximately 30 minutes.
Although most studies originally centered around the effects of hypothermia on brain tissue, it was soon discovered that acceptable ischemia times differed between organs, and that this difference was due to the baseline inherent ischemic tolerance of the specific organ tissue. Etz et al. (14) demonstrated in pigs that safe ischemic time for the spinal cord is close to that of the brain; however, the spinal cord has a baseline tolerance to ischemia that is about four times longer than that of the brain (20 versus 5 minutes). In other words, take away the baseline difference, and the time-temperature relationship for safe interruption of blood flow is the same for both the brain and the spinal cord. More precise ischemic times have not been borne out of the literature for the abdominal viscera, but clinical experience leads us to believe that the kidneys would have less ischemic tolerance than other intraabdominal viscera.
Rationale
Many advances both in surgical techniques and organ protection strategies have been made in aortic surgery over the past 35-40 years, yet repair of DTA and TAAA still remains a complex challenge to many cardiothoracic surgeons due to the high-risk nature of the patient population. Various techniques have been developed to aid in preserving end-organ function including clamp-and-sew, mild hypothermia with atriofemoral or femoral-femoral bypass, and DHCA. Most of the studies looking at outcome after these operations have focused mainly on cerebral and spinal cord events. As shown above, clearly some benefit is gained by suppressing metabolic activity even further using cooler temperatures. But, at what cost? Little data exists directly comparing these different surgical methods with respect to other end-organ functions, most notably intra-abdominal reversible adverse outcomes such as acute renal failure and liver failure. Therefore, this study aimed to determine the impact of distal ischemia time (DIT) and temperature on intra-abdominal reversible adverse outcomes (RAO) and permanent adverse outcomes (PAO) in patients undergoing DHCA versus non-DHCA repairs of DTA and TAAA.
Methods
A retrospective review of a single institutional database determined 262 consecutive patients who had undergone DTA and TAAA repair operations from 2002-2008. Twenty-two patients were excluded due to incomplete data or the presence of preoperative hemodialysis, resulting in 240 total patients [134 male (56%), mean age 62.6±13.2 years] with complete data for analysis. DIT was defined as circulatory arrest time plus the time required to restore distal perfusion, and if visceral artery reimplantation was necessary, that time interval was also added to the initial DIT. RAO studied were temporary postoperative hemodialysis, postoperative acute renal failure (more than 2× the admission creatinine level), or postoperative liver failure (more than 4× the admission hepatic transaminase level). PAO included were permanent hemodialysis in a previously non-dialyzed patient, paralysis, and mortality (death within 30 days of the operative procedure). Stroke was not included in PAO because it is not a result of distal ischemia.
Techniques
Patients who underwent DHCA were cooled using surface cooling blankets and perfusion cooling. If anticipated early, a minimum of 30 minutes of cooling was undertaken during the initial period of CPB. Adequate cerebral cooling was achieved if the jugular venous saturations were greater than 95% and the bladder temperature decreased to 12- 15 °C. If DHCA longer than 20 minutes was anticipated, the head was packed circumferentially in ice. Selective upper body perfusion was performed after the proximal anastomosis followed by perfusion warming at the end of the procedure maintaining a gradient less than 10 °C between the measured esophageal and blood temperatures. Motor evoked potential monitoring (MEPs), somatosensory evoked potential monitoring (SSEPs), cerebral spinal fluid (CSF) drainage via a CSF catheter, and preoperative steroid administration were all used liberally. Twenty-three patients in the DHCA group required reimplantation of at least 1 visceral vessel. Routinely, reimplantation of intercostal arteries was not performed, and generally, clamping of the left subclavian artery was avoided due to its collateral contributions. Non-DHCA techniques were executed with a cooling blanket to lower the bladder temperature to between 31 and 33 °C. Distal perfusion was added, usually after a period of distal ischemia during which an open anastomosis was completed. Postoperatively, CSF pressure was monitored and drained if the pressure exceeded 10 mmHg; methylprednisolone was continued for 3 days, and a high mean aortic pressure of at least 90 mmHg was maintained.
Results
DHCA techniques were carried out in 77 patients, and non-DHCA methods were used in 163 patients. Significant preoperative differences existed between the two groups with respect to age, body mass index, rates of reoperation, and rates of dissection. Thirty-day mortality rate was 7.1% (17/240): 6.1% in the non-DHCA group and 9.1% of DHCA patients (P=0.43). The paraplegia rate was 1.3% (3/240). In the entire cohort, 121 RAO developed in 98 patients, and 26 PAO developed in 24 patients. In the logistic regression analysis for the entire cohort, only RAO (OR 3.06; CI: 1.06-8.81) and preoperative creatinine >2.5 mg/dL (OR 4.34; CI: 1.45-13.02) were associated with developing PAO. In the entire cohort, RAO occurrence increased the probability of developing PAO (OR 4.69; CI: 1.78-12.37). There was also a significant trend toward an increased risk of PAO with higher numbers of RAO (probability of developing PAO with no RAO 4.3%, 1 RAO 16.7%, and 2 RAO 20.0%).
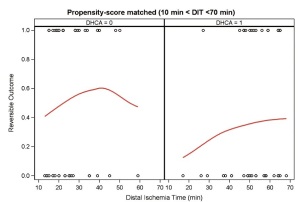
A propensity score matched analysis was performed to eliminate the unbalanced preoperative and intraoperative characteristics between the two groups. As DIT increased (10 min < DIT <70 min), a significantly lower rate of RAO was seen with DHCA compared with mild hypothermia (Figure 1). In the logistic regression analysis, variables associated with a statistically significant decrease in the odds of developing RAO included DHCA (OR 0.32; CI: 0.12-0.85) and stage II elephant trunk procedure (OR 0.22; CI: 0.06-0.79). To eliminate further confounders, the non-DHCA group was separated into a non-DHCA/elephant trunk group, and a non-DHCA/ non-elephant trunk group. Significantly decreased rates of acute renal failure (22% vs. 46%, P=0.03) and liver failure (17.8% vs. 39.3%, P=0.04) as well as a trend in decreased reversible hemodialysis rates (0% vs. 7.1%, P=0.07) were seen in the DHCA group versus the non-DHCA/non-elephant trunk group. Overall, the percentage of patients in the DHCA group with a RAO was 35.6% compared to 67.9% in the non-DHCA/ non-elephant trunk group (P<0.01). Logistic regression analysis found RAO (OR 14.10; CI: 1.70-117.02) and acute dissection (OR 23.5; CI: 1.05-528.02) to be significant predictors of PAO. Interestingly, the use of intraoperative blood products did not differ between the non-DHCA and DHCA groups (Table 1 ).

Full table
Impact and significance
Although substantial progress has been made in the surgical repair of DTA and TAAA, there still remains significant morbidity associated with these complex operations. This research underscores the importance of planning both preoperatively and intraoperatively for the optimal repair technique to decrease postoperative adverse events. The current study demonstrates a decreased rate of RAO (rates of acute renal failure and liver failure) in patients that received DHCA compared with matched patients having non-DHCA methods used to repair their DTA and TAAA. With the additional finding that RAO increased the probability of developing a PAO, these findings lend evidence to DHCA being not only a viable, but also possibly a superior option to mild hypothermia techniques. This also has increased financial impact, as decreased renovisceral events will lead to decreased lengths of stay and associated costs of patient care. Additionally, the advantages in this study found with stage II elephant trunk procedures go along with recent research showing decreased neurologic complications as well as acceptable renovisceral outcomes (15).
Proponents of mild hypothermia argue that coagulopathies are a major complication associated with the increased time spent on CPB to cool patients as well as the molecular level dysfunction associated with deep hypothermia. However, in this study both the DHCA and non-DHCA groups had statistically similar rates of red blood cell and fresh-frozen plasma transfusions, in line with other studies demonstrating a lack of increased hemorrhage (16,17). With standardized anti-fibrinolytic protocols in routine use, it is possible to gain the benefits of deep hypothermia without worsening coagulopathy.
Many limitations exist with this study. First, this is a retrospective study and hence has the same limitations associated with all studies of this type. The lack of randomization of patients introduces inherent bias to the conclusions reached. Additionally, the patient cohort was from a center specializing in aortic surgery as well as the implementation of DHCA. The same results may not be reproducible at surgical centers with less volume and less surgeon experience with deep hypothermia. Regardless, this study demonstrates the use of DHCA to be a safe technique with possibly superior renovisceral outcomes than those achieved with mild hypothermia with distal perfusion in DTA and TAAA surgery. Although DHCA may complicate and/or lengthen the nature of the operation, it provides a useful and safe tool to the aortic surgeon. Additionally, staged operations may prove helpful in reducing complications in patients with extensive aortic disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bigelow wg, Callaghan JC, Hopps JA. General hypothermia for experimental intracardiac surgery; the use of electrophrenic respirations, an artificial pacemaker for cardiac standstill and radio-frequency rewarming in general hypothermia. Ann Surg 1950;132:531-9.
- Lewis FJ, Taufic M. Closure of atrial septal defects with the aid of hypothermia; experimental accomplishments and the report of one successful case. Surgery 1953;33:52-9.
- Sealy WC, Brown IW Jr, Young WG Jr. A report on the use of both extracorporeal circulation and hypothermia for open heart surgery. Ann Surg 1958;147:603-13.
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63.
- Kirklin JW, Barratt-Boyes BG. eds. Hypothermia, circulatory arrest, and cardiopulmonary bypass. Cardiac surgery. 2nd ed. New York: Churchill Livingstone, 1993:61-127.
- Swain JA, McDonald TJ Jr, Griffith PK, et al. Low-flow hypothermic cardiopulmonary bypass protects the brain. J Thorac Cardiovasc Surg 1991;102:76-83; discussion 83-4.
- Michenfelder JD. The hypothermic brain. Anesthesia and the brain. New York: Churchill Livingstone, 1988:23-34.
- Ehrlich MP, McCullough JN, Zhang N, et al. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg 2002;73:191-7.
- McCullough JN, Zhang N, Reich DL, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg 1999;67:1895-9; discussion 1919-21.
- Ergin MA, Uysal S, Reich DL, et al. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999;67:1887-90; discussion 1891-4.
- Reich DL, Uysal S, Sliwinski M, et al. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J Thorac Cardiovasc Surg 1999;117:156-63.
- Fischer GW, Benni PB, Lin HM, et al. Mathematical model for describing cerebral oxygen desaturation in patients undergoing deep hypothermic circulatory arrest. Br J Anaesth 2010;104:59-66.
- Fischer GW, Lin HM, Krol M, et al. Noninvasive cerebral oxygenation may predict outcome in patients undergoing aortic arch surgery. J Thorac Cardiovasc Surg 2011;141:815-21.
- Etz CD, Luehr M, Kari FA, et al. Selective cerebral perfusion at 28 degrees C--is the spinal cord safe? Eur J Cardiothorac Surg 2009;36:946-55.
- LeMaire SA, Carter SA, Coselli JS. The elephant trunk technique for staged repair of complex aneurysms of the entire thoracic aorta. Ann Thorac Surg 2006;81:1561-9; discussion 1569.
- Harrington DK, Lilley JP, Rooney SJ, et al. Nonneurologic morbidity and profound hypothermia in aortic surgery. Ann Thorac Surg 2004;78:596-601.
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9.





