Patient selection for open thoracoabdominal aneurysm repair
The great Swiss surgeon Theodor Kocher [1841-1917] once said: “A surgeon is a doctor who can operate and who knows when not to” (1). Given the major impact of thoracoabdominal aortic replacement on human physiology, not to mention pre-existing co-morbidities and potential postoperative complications, it is absolutely mandatory to select operative candidates with great care for this intervention in order to achieve success. In each individual patient the operative risks have to be balanced against other treatment possibilities varying from medical therapy with antihypertensive drugs to the contemporary endovascular or hybrid treatment options. With improving diagnostic possibilities on the one hand and an aging population on the other hand, more and more patients with limited physiologic reserve are being referred for possible treatment. Therefore the decision to intervene, either with conventional open surgery, endovascular procedure or a combination of both, should be based on a predicted operative risk that is lower than the risk of optimal medical management alone. It is the clinical task of the surgeon and anaesthesiologist to select those candidates for whom the operative risks are justified. Objective selection criteria like those accepted for aneurysms of the thoracic aorta (2) could be of great value for this together with solid clinical judgment.
Operative indications for open thoracoabdominal aortic repairOther Section
- Operative indications for open thoracoabdominal aortic repair
- Sizing
- Size criteria
- Concerns regarding rapid growth
- Preoperative patient evaluation
- Conclusions
- Acknowledgements
- References
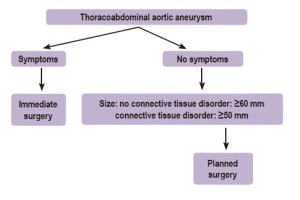
Since the outcome of open thoracoabdominal repair is, amongst other things, linked to the initial clinical presentation, early diagnosis is of paramount importance. One has to make a clear distinction between symptomatic and asymptomatic patients. Most patients with thoracoabdominal aortic aneurysms experience no or only minor symptoms and the aneurysm is often found incidentally during radiologic procedures for other indications. Symptomatic patients are certainly in the minority when we do not take into account the vague back pain that is difficult to be related solely to the aneurysm. If frank symptoms occur, they usually can be attributed to leakage and its hemodynamic consequences: hypovolemic shock and death if no intervention is performed. Massive haemoptysis or hematemesis are signs of a fistulisation between the aneurysm and the respiratory or gastrointestinal tract respectively. All ruptured and symptomatic thoracoabdominal aortic aneurysms should be operated on immediately, regardless of size, if the general condition of the patient allows it. Rupture may be impending, meaning imminent or threatening; or actual, meaning certain and visualised at surgery. Mortality after surgical treatment of ruptured thoracoabdominal aneurysms is high, with a rate of 54% in the United States between 1988 to 1998 (3). Rupture also has a serious negative impact on late survival (4,5). Symptomatic thoracoabdominal aneurysms without the presence of rupture or near rupture on the other hand, can be attributed to the aneurysm compressing vital organs such as the trachea, left main bronchus and lung (dyspnoea or stridor), the oesophagus (dysphagia), the left laryngeal recurrent nerve (hoarseness) or parts of the heart (decompensation). Under these circumstances surgery can be planned without the necessity to rush in immediately.
Since the majority of patients with thoracoabdominal aortic aneurysms are asymptomatic, size becomes the single most commonly used criterion to decide whether or not to intervene in order to prevent rupture. Based on Laplace´s law the aneurysmal diameter becomes the most important determinant of the probability of rupture. The Yale group has provided us with lifetime observational evidence on asymptomatic patients showing that the risk of dissection or rupture increases with the aneurysm size (6). Davies et al. have shown that at the size of 60 mm, the risk of rupture, dissection, and death increases in a stepwise fashion (7). The complication rate increases exponentially beyond this critical point. The size criteria is confirmed by the ACCF/AHA guidelines (8) as level C evidence, Class I recommendation. Therefore sizing of the thoracoabdominal aorta is crucial. It has to be done correctly in order to interpret these data properly and one has to know what the normal size of the aorta is. More than two decades ago the normal diameters of the different parts of the thoracic aorta have been described based on computed tomography (CT) and chest X-ray (9). Now we know that age, sex, body surface area as well as the location of the aortic measurement, the method of the measurement and the type of imaging technique used, all influence the normal aortic diameter during each cardiac cycle.
SizingOther Section
- Operative indications for open thoracoabdominal aortic repair
- Sizing
- Size criteria
- Concerns regarding rapid growth
- Preoperative patient evaluation
- Conclusions
- Acknowledgements
- References
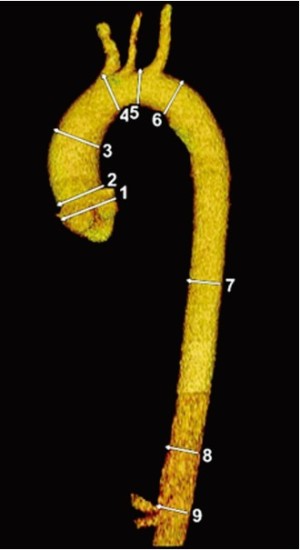
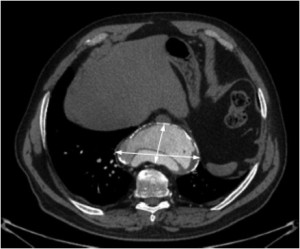
In 2010 the ACCF/AHA guidelines delineated important recommendations with regard to aortic sizing (8) with CT or magnetic resonance imaging (MRI). These recommendations included sizing at reproducible anatomic landmarks: mid aortic arch between left subclavian and left common carotid artery, proximal descending thoracic aorta, mid descending thoracic aorta, aorta at diaphragm 2 cm above celiac axis origin, and abdominal aorta at celiac axis origin (Figure 1). Additional important levels are the abdominal aorta at renal artery origin, the infrarenal diameter, the abdominal aorta 2 cm above bifurcation, at the bifurcation, and both common iliac artery diameters. The external diameter should be measured perpendicular to the axis of blood flow with reporting of abnormalities of aortic morphology (Class I recommendation, Level evidence C). In case of aortic dissection, intraluminal clot, and aortic wall inflammation, it becomes even more important to use external aortic diameters rather then the lumen size. Axial sections remain the mainstay of CT images interpretation but two- and three-dimensional reconstructions may be helpful in describing the aneurysmal contours. It is very important to measure perpendicular to the centreline or axis of the aorta, especially in very tortuous aortas (Figure 2). Hemodynamic forces resulting from cardiac contraction (10) but also the thoracic aortic pulsatility, e.g. during hypovolemia, naturally results in significant conformation changes of the aorta and its size (11).
Size criteriaOther Section
- Operative indications for open thoracoabdominal aortic repair
- Sizing
- Size criteria
- Concerns regarding rapid growth
- Preoperative patient evaluation
- Conclusions
- Acknowledgements
- References
Asymptomatic patients with a maximal aneurysmal diameter of twice the size of a normal, contiguous aortic segment or greater than 60 mm are potential candidates for surgical resection unless medical comorbidities prohibit this (12-15). Since thoracoabdominal aneurysms classically have an hourglass configuration with adjacent consecutive smaller and wider areas, the maximal diameter is decisive. Smaller than 60 mm size criteria can be applied to patients with connective tissue disorders of which Marfan syndrome is the prototype. The size standards and the relation with symptoms are depicted in Figure 3. In 2006 Davies et al. (16) introduced the risk of aortic complication (rupture and/or dissection and/or death) related to the body surface area. They stratified patients into three risk categories (low, medium and high risk) based on aortic size index depending on body surface area on the one hand and maximal aortic diameter on the other hand. The authors suggest to operate before a patient enters the zone of moderate risk.
Concerns regarding rapid growthOther Section
- Operative indications for open thoracoabdominal aortic repair
- Sizing
- Size criteria
- Concerns regarding rapid growth
- Preoperative patient evaluation
- Conclusions
- Acknowledgements
- References
The descending thoracic or thoracoabdominal aorta grows faster than the ascending aorta and arch (0.19 versus 0.07 cm/year) while abdominal aneurysms have a mean growth rate of 0.28 cm/year (7). When dissection has occurred the yearly growth rate of the thoracic aorta increases to 0.28 cm/year and this warrants aggressive surveillance (6). When the aneurysm is expanding rapidly which is defined as a yearly increase in size of more than 1 cm, symptoms like back pain may become more obvious and severe. Consecutive CT scans with short time interval may elucidate this rapid increase in size. In dissected aortas in which the false lumen is partially or completely closed or thrombosed, growth rate is lower compared to situations in which the false lumen remains patent (17-19). For accurate assessment of rapid growth, comparisons of the sizes have to be made exactly at the same level of the aorta (see guidelines of the ACCF/AHA) (8). Quick expansion often occurs as a consequence of acute dissection, contained rupture, or when there is a mycotic component.
Preoperative patient evaluationOther Section
- Operative indications for open thoracoabdominal aortic repair
- Sizing
- Size criteria
- Concerns regarding rapid growth
- Preoperative patient evaluation
- Conclusions
- Acknowledgements
- References
All planned interventions on the thoracoabdominal aorta should be preceded by a profound assessment of all major organ systems, such as cardiac, pulmonary and renal, to outline the patient’s overall health and physiologic reserve for each organ system specifically. The rationale for this is that most patients with thoracoabdominal aortic aneurysms have concomitant central and peripheral atherosclerotic lesions, aside from those with connective tissue disorders. Next, associated medical problems are commonplace in patients with aneurysmal disease in general and therefore one must focus during the preoperative evaluation on a multitude of organs that are potentially at risk during and after surgery. For example the majority of patients have coexisting hypertension: 76% in Coselli’s series (20), 73% in Safi’s series (21), and 66% in our own (22). Obviously all comorbidities may increase the surgical risk (23) and the negative impact of coronary artery, pulmonary and renal disease on survival is of paramount importance and has been well documented (4). In symptomatic patients with leaking aneurysms there is little or no time to perform more than the basic evaluation and few patients have time to undergo extensive cardiac assessment. Optimal communication between the anaesthetist, the surgeon and the perfusionist are important to delineate an overall strategy for perioperative care (9).
Respiratory failure with prolonged ventilatory support and the need for tracheotomy is the most common complication after open thoracoabdominal replacement (24). The anaesthetist may be faced with an altering hemodynamic status during clamping, possible myocardial ischemia and single lung ventilation in case of left heart bypass use causing right-to-left shunt through the non-ventilated left lung with potentially hypoxemia. Chest radiography is of little value in making a diagnosis and will show an enlarged aortic shadow, with or without evidence of associated pulmonary or cardiac disease. Tracheal or left main bronchus compression and deviation is occasionally evident but CT-scan certainly will give more information in these circumstances. Nevertheless it is absolutely necessary to have a baseline chest X-ray for comparison in the early postoperative period or for the exclusion of other causes of the patient’s symptoms. Accordingly lung function testing should be performed routinely together with blood gas analysis during rest. Since both abdomen and thorax are opened during the repair with circumferential division of the diaphragm and transection of the costal margin, patients are particularly prone to developing postoperative respiratory failure. Needless to say that the majority of the patients have a smoking history and smoking should be absolutely discontinued before surgery. Also chronic obstructive pulmonary disease is commonplace in this patient group: 40% in the series of LeMaire et al. (25) and it is even associated with growth and rupture of the aneurysm (26). Pulmonary reserve may be optimized by cessation of smoking, the use of bronchodilators and a comprehensive exercise programme. The mobility of the vocal cords should be evaluated preoperatively because the left recurrent laryngeal nerve may be damaged during clamping, sewing or simply due to the use of electro-cautery (27). However, its function may already be compromised by pressure or stretching by the aneurysm itself.
Because the incidence of coronary artery disease is not
negligible in patients with thoracoabdominal aneurysms
[36% in the series of Coselli et al. (20), 27% in the series of
Safi et al. (21)], we suggest to complete the following cardiac
screening tests in elective cases:
(I) Electrocardiography to detect cardiac ischemia,
previous myocardial infarction, serious rhythm disturbances,
ventricular hypertrophy.
(II) Transthoracic echocardiography (TTE) to
evaluate the left and right ventricular function, valve (dys)
function, size of the aortic root and to visualize possible
compression on the cardiac chambers. Transoesophageal
echocardiography should only be used when TTE
information is insufficient (apart from its intraoperative
use which is almost mandatory). Stress-related severe
hypertension during this examination, especially during the
insertion of the echo probe, should be anticipated.
(III) Cyclo-ergometry test is dangerous in case of large
aneurysms and should not be performed. Cardiologists
should be aware of that since the decision to perform this
test is often made almost automatically and independently
by cardiac laboratory technicians.
(IV) Dobutamine-stress-echocardiography or
dipyridamole-thallium imaging may demonstrate ischaemic
zones necessitating coronary angiography.
(V) Coronary angiography is only performed in
patients with symptoms of cardiac ischemia and positive
testing for cardiac ischemia, in patients who underwent
previous coronary artery bypass grafting or coronary stent
implantation and in patients with previous myocardial
infarction. Significant coronary artery disease should
be addressed by angioplasty or coronary artery bypass
grafting before aneurysm repair (20). The use of the
left internal mammary artery may cause serious problems
if the left subclavian artery must be clamped during
thoracoabdominal replacement.
Furthermore a complete blood examination including coagulation parameters should be checked preoperatively. Coagulation should be corrected preoperatively in order to safely insert a spinal catheter according to the current guidelines (28). Patients with anaemia can be prepared with iron supplements and erythropoietin several days before surgery to increase red cell mass (29). All this will reduce or even abolish the burden of red blood cell transfusion.
Since stroke can occur after thoracoabdominal aortic aneurysm repair for instance as a consequence of aortic arch clamping, duplex scanning of the cerebral vessels in elective patients can demonstrate the presence of concomitant stenoses in the carotid and/or vertebral arteries. Needless to say, patients with a history of stroke should undergo thorough neurologic evaluation, possibly including CT scanning of the brain.
Postoperative renal failure is predictive of poor outcome (30) . Unfortunately, many patients with thoracoabdominal aortic aneurysms have involvement of the renal arteries within the extent of the aneurysm itself or have intrinsic renal artery stenoses (atherosclerosis or dissection), which may result in renal impairment. Therefore measurement of serum creatinine or glomerular filtration rate is necessary. The use of angiographic contrast dye shortly before surgery in elective cases should be avoided. In scheduled cases the repair should be deferred until laboratory values are normalised. The operative planning also includes the evaluation of the status of the peripheral vessels for signs of peripheral vascular disease, which can be defined either by angiography, CT scan, or ultrasound. An overview of the suggested preoperative examinations in elective cases is given in table 1.
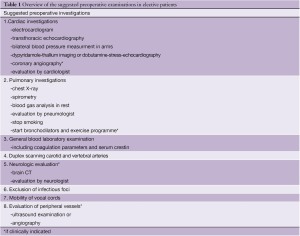
Full table
ConclusionsOther Section
- Operative indications for open thoracoabdominal aortic repair
- Sizing
- Size criteria
- Concerns regarding rapid growth
- Preoperative patient evaluation
- Conclusions
- Acknowledgements
- References
Surgeons should realize that an open thoracoabdominal replacement is a major cardiovascular intervention affecting multiple organ systems and may be complicated by serious intra- and postoperative problems. Through better selection, evaluation and preparation of surgical candidates, results can be improved. This alertness, together with meticulous surgery, has resulted in a drastic reduction of all major complications in large aortic centers, but one should realize that all that glitters is not gold (31). The decision to replace the thoracoabdominal aorta is not a minor one and has major implications. Therefore age, comorbidities and life expectancy should also be taken into consideration. Above all, it is important to realise that “not all that is technically feasible is in the patient’s best interest” {Mark M. Ravitch [1910-1989]}.
AcknowledgementsOther Section
- Operative indications for open thoracoabdominal aortic repair
- Sizing
- Size criteria
- Concerns regarding rapid growth
- Preoperative patient evaluation
- Conclusions
- Acknowledgements
- References
Disclosure: The authors declare no conflict of interest.
ReferencesOther Section
- Operative indications for open thoracoabdominal aortic repair
- Sizing
- Size criteria
- Concerns regarding rapid growth
- Preoperative patient evaluation
- Conclusions
- Acknowledgements
- References
- Knut Haeger. The illustrated history of surgery. NY: Harold Starke Publishers Limited 1989.
- Elefteriades JA. Indications for aortic replacement. J Thorac Cardiovasc Surg 2010;140:S5-9; discussion S45-51.
- Cowan JA Jr, Dimick JB, Wainess RM, et al. Ruptured thoracoabdominal aortic aneurysm treatment in the United States: 1988 to 1998. J Vasc Surg 2003;38:319-22.
- Schepens MA, Kelder JC, Morshuis WJ, et al. Long-term follow-up after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2007;83:S851-5; discussion S890-2.
- Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and longterm results of operations in 605 patients. J Vasc Surg 1986;3:389-404.
- Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 1997;113:476-91; discussion 489-91.
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27; discussion 27-8.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/ SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369.
- Silvay G, Stone ME. Repair of thoracic aneurysms, with special emphasis on the preoperative work-up. Semin Cardiothorac Vasc Anesth 2006;10:11-5.
- Muhs BE, Vincken KL, van Prehn J, et al. Dynamic cine-CT angiography for the evaluation of the thoracic aorta; insight in dynamic changes with implications for thoracic endograft treatment. Eur J Vasc Endovasc Surg 2006;32:532-6.
- Jonker FH, van Keulen JW, Schlosser FJ, et al. Thoracic aortic pulsatility decreases during hypovolemic shock: implications for stent-graft sizing. J Endovasc Ther 2011;18:491-6.
- Thoracoabdominal aortic aneurysms with special reference to technical problems and complications. “The Marstrand Workshop-group”. Eur J Vasc Surg 1993;7:725-30.
- Coselli JS, LeMaire SA. Surgical techniques. Thoracoabdominal aorta. Cardiol Clin 1999;17:751-65.
- Sternbergh WC 3rd, Gonze MD, Garrard CL, et al. Abdominal and thoracoabdominal aortic aneurysm. Surg Clin North Am 1998;78:827-43, ix.
- Schepens MA, Verrelst PA, Ranschaert W, et al. Indications for thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg 2010;140:S121-4; discussion S142-S146.
- Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169-77.
- Sueyoshi E, Sakamoto I, Uetani M. Growth rate of affected aorta in patients with type B partially closed aortic dissection. Ann Thorac Surg 2009;88:1251-7.
- Song SW, Chang BC, Cho BK, et al. Effects of partial thrombosis on distal aorta after repair of acute DeBakey type I aortic dissection. J Thorac Cardiovasc Surg 2010;139:841-7.
- Fattori R, Bacchi-Reggiani L, Bertaccini P, et al. Evolution of aortic dissection after surgical repair. Am J Cardiol 2000;86:868-72.
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4; discussion S890-2.
- Safi HJ, Estrera AL, Azizzadeh A, et al. Progress and future challenges in thoracoabdominal aortic aneurysm management. World J Surg 2008;32:355-60.
- Schepens MA, Defauw JJ, Hamerlijnck RP, et al. Surgical treatment of thoracoabdominal aortic aneurysms by simple crossclamping. Risk factors and late results. J Thorac Cardiovasc Surg 1994;107:134-42.
- Coady MA, Ikonomidis JS, Cheung AT, et al. Surgical management of descending thoracic aortic disease: open and endovascular approaches: a scientific statement from the American Heart Association. Circulation 2010;121:2780-804.
- Lemaire SA, Rice DC, Schmittling ZC, et al. Emergency surgery for thoracoabdominal aortic aneurysms with acute presentation. J Vasc Surg 2002;35:1171-8.
- LeMaire SA, Miller CC 3rd, Conklin LD, et al. Estimating group mortality and paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2003;75:508-13.
- Coady MA, Rizzo JA, Goldstein LJ, et al. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 1999;17:615-35; vii.
- Ishii K, Adachi H, Tsubaki K, et al. Evaluation of recurrent nerve paralysis due to thoracic aortic aneurysm and aneurysm repair. Laryngoscope 2004;114:2176-81.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010;35:64-101.
- Society of Thoracic Surgeons Blood Conservation Guideline Task Force, Ferraris VA, Brown JR, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011;91:944-82.
- Schepens MA, Defauw JJ, Hamerlijnck RP, et al. Risk assessment of acute renal failure after thoracoabdominal aortic aneurysm surgery. Ann Surg 1994;219:400-7.
- Derrow AE, Seeger JM, Dame DA, et al. The outcome in the United States after thoracoabdominal aortic aneurysm repair, renal artery bypass, and mesenteric revascularization. J Vasc Surg 2001;34:54-61.