Thoracoabdominal aortic aneurysm repair with a branched graft
Introduction
Many aspects of open thoracoabdominal aortic aneurysm (TAAA) repair are individualized according to patient-specific factors related to the type and extent of disease, comorbid conditions, and physiological reserve. One example of how surgeons can individualize the technical approach to this operation is the use of a prefabricated aortic graft with four side branches designed for reattaching the celiac axis, superior mesenteric artery (SMA), and both renal arteries (1-4). Using this branched graft in TAAA repairs is ideal when one of two conditions are met: (I) The patient has a connective tissue disorder (e.g., Marfan syndrome, Loeys-Dietz syndrome), and aortic tissue that remains after the procedure will be prone to aneurysmal dilatation, pseudoaneurysm formation, and rupture, eventually necessitating reintervention (5-7); or (II) the origins of the patient’s visceral vessels are far enough apart that an island patch reimplantation is not desirable.
The ultimate goal of these operations is to balance the need to resect and replace as much diseased aortic tissue as possible with the need to protect the spinal cord and other organs and, thereby, prevent postoperative complications. Our strategies for organ protection have been described in detail elsewhere (8-14). To protect the spinal cord, we employ mild passive hypothermia, cerebrospinal fluid (CSF) drainage, left heart bypass (LHB), sequential cross-clamping, and selective reimplantation of intercostal or lumbar arteries (9-11,14). The renal arteries are perfused with cold crystalloid solution to protect the kidneys from ischemic damage (8,12,13). Perfusing the celiac axis and the SMA with isothermic blood from the LHB circuit minimizes the duration of abdominal-organ ischemia.
Operative techniques
Preoperative planning
The patient’s history and physical examination findings are thoroughly reviewed, along with pertinent findings from the preoperative evaluation. The patient’s age, history of tobacco use, ejection fraction, pulmonary function, and kidney function, as well as the status of the carotid and coronary arteries, all factor into intraoperative decisions regarding patient management and surgical technique (10,14). The patient’s computed tomographic angiography scans are reviewed to plan various technical aspects of the procedure. The scans are checked for relevant anatomic variants, such as a retroaortic left renal vein. The diameter of the aorta in its nonaneurysmal portions—often just distal to the left subclavian for the proximal anastomotic site and at the aortic bifurcation for the distal anastomotic site—provides an idea as to the size of the Dacron graft that will be needed. Potential clamping and cannulation sites are evaluated. For example, the planned aortic cannulation site for LHB should be in a region that is free from extensive mural thrombus. The spatial relationships between the celiac, SMA, and renal arteries are assessed. When the origins of the branch arteries are substantially displaced away from one another, using a branched graft will enable their reattachment without leaving behind a large patch of aortic tissue (5). In patients with aortic dissection, the configuration of the dissecting membrane and the relationships between the true lumen, false lumen, and branch arteries are evaluated. Furthermore, areas of stenosis at the origins of the visceral and renal arteries are identified because they may need endarterectomy, stenting, or both (15). Although the final decision with regard to any of the aforementioned technical considerations is made intraoperatively on the basis of operative anatomy, it is very useful to have considered these details in advance.
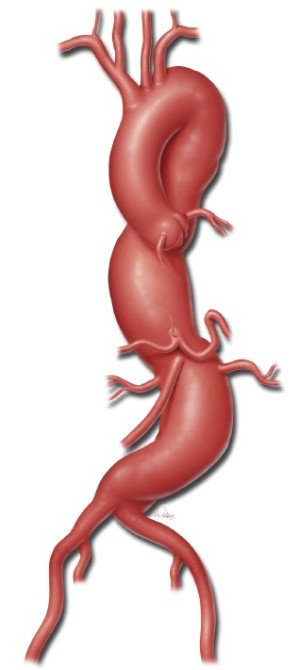
To illustrate the use of a branched graft, we will describe in detail the procedure for performing a Crawford extent II TAAA repair. A typical patient undergoing an extent II repair has an aneurysm extending from the left subclavian artery down to the aortic bifurcation (Figure 1). In this particular case, there is a neck of proximal descending aorta distal to the left subclavian artery that can be clamped. Note also that the celiac axis, SMA, and right and left renal arteries are spread relatively far apart along the circumference of the aneurysmal aorta.
Anesthesia considerations and patient positioning
The patient is placed supine on top of a beanbag on the operating table. Both arms are placed on arm boards on either side of the table. A right radial or brachial arterial line is placed by the anesthesiologist. A large-bore peripheral intravenous line is likewise placed at this time. After intravenous sedative and muscle relaxant are administered, the patient is intubated with a double-lumen endobronchial tube for later single right-lung ventilation, and general anesthesia is induced.
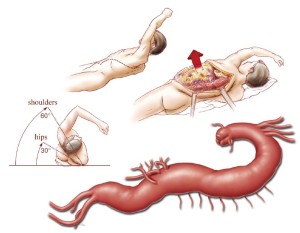
The patient is placed in a right lateral decubitus position with both hips and knees flexed for spinal drain placement by the anesthesiologist. An axillary roll is placed between the bed and the patient, just under the right axilla. The spinal drain is placed at the L3-4, L4-5, or L5-S1 level. The CSF pressure is maintained at less than 10 mmHg, but the amount drained is limited to no more than 10 mL/hour. The spinal drain is secured with tape to the right side of the patient’s back, and the patient is repositioned such that the upper body is at a 60-degree angle and the hips are at a 30-degree angle to the horizontal. This allows for access to both groins in case there is a need for access to the femoral arteries. The beanbag is suction-deflated and made firm against the patient’s body to keep the patient properly positioned. The patient’s left arm is placed on top of an elevated arm board and held at an angle above the shoulders in a freestyle swimming stroke position (Figure 2). The correct position of the double-lumen endobronchial tube is confirmed with a flexible bronchoscope after final positioning. The patient’s left chest and back, abdomen, groins, and upper thighs are prepared and draped in a sterile fashion. An adhesive antimicrobial drape is placed over all exposed skin. Prophylactic broad-spectrum intravenous antibiotics are given within 1 hour of skin incision. The patient’s mean arterial pressure (MAP) is maintained in the range of 70 to 90 mmHg throughout the case.
Incision and aortic exposure
A sigmoid-shaped skin incision is made from just posterior to the inferior aspect of the left scapula, curving along the 7th rib and across the costal margin toward a point about an inch to the left of the umbilicus. To avoid creating areas of skin necrosis, gentle curves are followed instead of sharp angles when the incision is made. When a repair will involve the iliac arteries, the incision may be extended inferiorly around the umbilicus and into the midline to just above the pubic symphysis.
The latissimus dorsi is divided, followed by the serratus anterior, to provide entry into the plane of the rib cage. Ribs may be counted downward from the apex or upward from the lower ribs to confirm appropriate interspace entry into the left pleural cavity. For most extent II thoracoabdominal repairs, the 6th intercostal space is the best entry point; the 5th intercostal space is occasionally used, such as when there is an especially large aneurysm involving the distal aortic arch and proximal descending thoracic aorta. The anesthesiologist is asked to render the left lung atelectatic at this point and to commence single right-lung ventilation. Then, the intercostal muscle is detached from the rib below the space chosen, and this incision is carried posteromedially toward the spine and anteriorly to the costal margin. The costal margin is divided, and a portion of it is resected to prevent overlap during reapproximation of the separated ribs at the end of the case. A short posterior segment of the rib may be resected to gain additional exposure.
The diaphragm is divided at the costal margin to expose the peritoneum. A fold of the peritoneum is palpated to make sure that the stomach, transverse colon, and liver are not adherent; the peritoneum is then carefully opened. The abdominal portion of the thoracoabdominal incision is then opened under direct vision to avoid inadvertent injury to intra-abdominal organs. Left medial visceral rotation is then carried out by entering the avascular plane along the line of Toldt. Retroperitoneal fibro-fatty tissues are separated from the inferior portion of the left hemidiaphragm and the anterior aspect of the left psoas muscle. Care is taken to identify and preserve the left ureter and gonadal vein. If a retroaortic left renal vein is present, it is preserved when possible; however, during extent II repairs, it is often necessary to divide the vein and later reconstruct it with an interposition graft. The left hemidiaphragm is divided circumferentially, leaving a 3- to 4-cm rim attached to the rib cage, from the left costal margin to the left crus. Retraction sutures are placed along the edge of the divided diaphragm on the cardiac side. The intra-abdominal aorta is exposed by using electrocautery to divide the retroaortic fibro-fatty tissues between the jaws of a right-angle clamp. The left renal artery and the aortic bifurcation are exposed.
A large Richardson retractor, together with an upper hand retractor, is secured to the table-mounted ether screen to hold the upper rib cage open, and a table-mounted selfretaining retractor with bladder blades pulls the lower ribs posteriorly and to the left. This provides generous exposure for the repair (Figure 2). The proximal and distal clamp sites are developed by using low-voltage electrocautery and a pair of long Metzenbaum scissors. Care is taken to identify and preserve the left recurrent laryngeal nerve and the left phrenic nerve. Sometimes, the ligamentum arteriosum must be divided to improve mobilization around the proximal descending thoracic aorta and distal transverse aortic arch. This maneuver is particularly useful when the proximal clamp site needs to be positioned on the aortic arch, between the left common carotid and left subclavian arteries. The distal clamp site is positioned at the level of the left pulmonary hilum. It is important to stay anterior to the hemiazygos vein and intercostal veins, and it may be necessary to ligate some intercostal arteries at the clamp site with medium-sized clips. Likewise, the adjacent esophagus is identified and dissected away from the aortic clamp site.
Cannulation and perfusion setup
Either the superior or the inferior left pulmonary vein can be used as an atrial cannulation site for LHB (9). The left lung is retracted posterolaterally with a moist laparotomy pad and a deep Deaver retractor. The pericardium is opened near the pulmonary veins, away from the phrenic nerve. A 3-0 pledgeted polypropylene suture is placed in the origin of the vein in a mattress fashion. At the aortic cannulation site, a purse-string suture is placed in the same manner whether the site is in the distal descending thoracic aorta or the proximal abdominal aorta (i.e., proximal to the left renal artery origin).
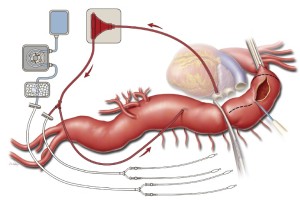
Heparin is administered intravenously at a dose of 1 mg/kg. A 24-French angled-tip cannula is placed in the left atrium via a pulmonary venotomy, secured with the previously placed purse-string suture and a Rummel tourniquet, and connected to the drainage line of the LHB circuit. This cannula is secured to the patient’s skin with a towel clip placed in the left subcostal area. A 20-French angled-tip cannula is placed in the distal aorta and connected to the inflow line of the LHB circuit (Figure 3). This cannula is secured to the patient’s skin with a towel clip placed near the umbilicus. The inflow line of the LHB circuit has a Y-connector attached that splits pump return between the line going to the distal aortic cannula and another line leading to two 9-French Pruitt balloon-tipped perfusion catheters for later delivery of selective visceral perfusion to the celiac artery and SMA (Figure 3). This selective visceral perfusion line remains clamped while distal aortic perfusion is being provided. A separate cold crystalloid renal perfusion circuit is set up with another two 9-French Pruitt balloon-tipped perfusion catheters attached to the end of its line for later administration of 4 ℃ lactated Ringer’s solution with mannitol (12.5 gm/L) and methylprednisolone (125 mg/L) to the renal arteries (Figure 3) (8,12,13).
Aortic reconstruction
After LHB is initiated at a flow rate of 500 mL/min, a straight, padded aortic cross-clamp is applied just distal to the left subclavian artery. This clamp is secured with a towel clip to the skin of the upper chest on the superior side of the incision. The proximal descending thoracic aorta is compressed manually with a laparotomy pad to displace blood distally; then a Crafoord clamp is applied across the aorta at the junction of the upper and middle thirds of the descending thoracic aorta (Figure 3). If the aortic segment between the two clamps remains pressurized, this may indicate that the proximal clamp has not been applied all the way across; the proximal clamp may have to be reapplied or a second clamp may have to be placed to achieve proximal control.
After the aorta is clamped, LHB flow is increased toward a target between 1.5 and 2.5 L/min to keep the patient’s MAP around 80 mmHg. The aortic segment between the two clamps is then opened longitudinally by using electrocautery, and its edges are retracted laterally with 0 silk stay sutures. Shed blood is collected by a cell-saver system for filter processing, and the washed red cells are collected and auto-transfused back into the patient. In cases involving aortic dissection, the dissecting membrane is excised. Brisk back-bleeding from intercostal arteries is controlled with 2-0 silk suture ligatures placed in a figureof- eight fashion; this minimizes blood loss, improves visualization, and prevents shunting of blood away from the spinal circulation. A cuff of proximal descending thoracic aorta about 2-3 centimeters distal to the proximal aortic clamp is cut transversely and carefully separated from the underlying esophagus by using electrocautery; this important step prevents the incorporation of the esophagus into the suture line when the proximal anastomosis is sewn.
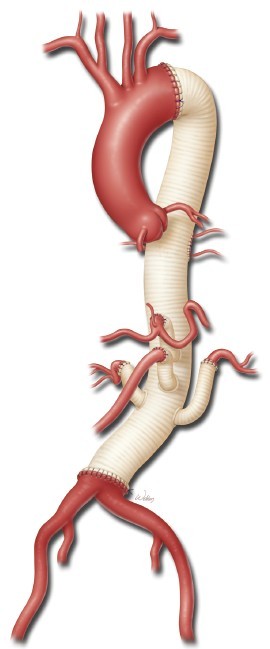
The size of the four-branched aortic graft is chosen partially on the basis of the aortic diameters measured at the planned proximal and distal anastomosis sites on preoperative imaging, but the choice is largely based on visual inspection of the aorta intraoperatively. Usually, a 24-, 26-, or 28-mm graft is used. The four branch grafts arise from the distal third of the main body of the graft, and they include two anteriorly-placed 10-mm branches that are about 1 cm apart (corresponding to the celiac axis and the SMA) and two 8-mm side branches placed about 1 cm distal to the second 10-mm branch on either side of the graft (corresponding to the renal arteries). The proximal end of the aortic graft is trimmed to the appropriate length to match the distance from the proximal anastomotic site to the location of the visceral arteries distally (inset, Figure 4). Ideally, the origin of each branch graft is positioned slightly inferior to the origin of its paired artery. This facilitates the formation of gentle curves in the branch grafts that help prevent them from becoming kinked. This positioning can be rapidly achieved by stretching the graft so that it is taut, lining up the origin of the celiac graft with the origin of the left renal artery, and cutting the proximal end of the aortic graft at the point where it reaches the proximal anastomosis site.
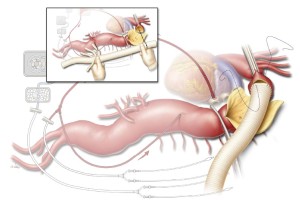
The anastomosis between the aortic graft and the proximal aortic cuff is sewn in an end-to-end configuration with a continuous 3-0 or 4-0 polypropylene suture (Figure 4). Although 3-0 suture is used in most cases, 4-0 suture is preferred when the aortic tissue is extremely fragile (e.g., in some patients with Marfan syndrome). The suture line is often reinforced with a circumferential layer of pledgeted 3-0 or 4-0 polypropylene sutures in an interrupted mattress fashion. After the proximal anastomosis is completed, the proximal aortic clamp is left in place if it is positioned distal to the left subclavian artery. However, if the clamp had been placed proximal to the left subclavian artery, the patient is placed in Trendelenburg position and the clamp on the left subclavian artery is removed to de-air the proximal portion of the graft; the aortic clamp is then moved onto the graft to restore blood flow to the left subclavian artery.
Left heart bypass is stopped at this point, and the tubing going to the distal aortic cannula is clamped. Simultaneously, the distal aortic clamp is removed and the aorta is opened longitudinally down to the aortic bifurcation; care is taken to cut posterior to the origin of the left renal artery. Once again, 0 silk retraction sutures are applied to both edges of the opened aorta along its length to provide wide exposure of the lumen. Shed blood continues to be collected with the cell-saving system. In cases of aortic dissection, the exposed dissecting membrane is excised. Briskly back-bleeding intercostal and lumbar arteries are suture ligated in a figure-of-eight fashion with 2-0 silk sutures to prevent shunting of blood away from the spinal circulation. A briskly back-bleeding inferior mesenteric artery may likewise be ligated at this point. The origins of the visceral arteries off of the aneurysmal native aorta are identified and inspected for significant stenosis, calcification, or dissection. Such lesions can be managed by performing an endarterectomy, fenestrating a dissecting membrane, or placing uncovered balloon-expandable stents (6- or 7-mm diameter, 14- or 15-mm length) (15). The celiac artery and SMA are cannulated with the 9-French Pruitt visceral perfusion catheters connected to the inflow line of the LHB circuit, and selective perfusion is delivered at a flow rate of approximately 200 mL/min. The renal arteries are likewise cannulated with the 9-French Pruitt catheters, and boluses of the cold crystalloid solution are administered every 6 to 10 min at volumes generally ranging between 200 and 400 mL (Figure 5) (8,12,13). Nasopharyngeal temperature is monitored closely; the temperature usually drifts down into the range of 32 to 34 ℃. To avoid hypothermia-induced arrhythmias, additional boluses of cold crystalloid renal perfusion are not administered if the nasopharyngeal temperature is at or below 32 ℃.
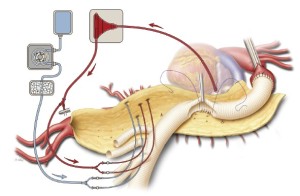
The remaining patent intercostal and lumbar arteries are carefully inspected, particularly between T7 and L1; those that are large and have little back-bleeding are chosen for reimplantation. An opening in the side of the graft is cut to size, and a side-to-side anastomosis is created with continuous 3-0 or 4-0 polypropylene suture (Figure 5). The anastomosis is constructed in a manner that minimizes the amount of aortic tissue within the reattached patch. Fragile areas within the anastomosis are selectively reinforced with 3-0 or 4-0 pledgeted polypropylene sutures in a mattress fashion.
After the patch reimplantation of the intercostal arteries is completed, whenever possible, the proximal aortic cross-clamp is moved down the aortic graft to a position immediately distal to the intercostal patch (Figure 6). This allows for reperfusion of the reimplanted intercostal arteries as part of the spinal protection strategy known as sequential cross-clamping. The distal end of the aortic graft is trimmed to the appropriate length, and the distal anastomosis is performed in an end-to-end configuration with a continuous 2-0, 3-0, or 4-0 polypropylene suture (depending on the quality of the tissue) (Figure 6). The circumference of the distal anastomosis is selectively reinforced with pledgeted 3-0 polypropylene sutures in an interrupted mattress fashion.
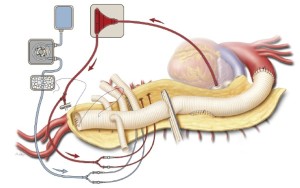
The aortic graft and its four branches are allowed to fill with blood from the distal anastomosis. Hemostat clamps are placed across each branch of the graft; then the patient is placed in Trendelenburg position, and the aortic cross-clamp is slowly removed to reestablish blood flow to the pelvis and both lower extremities (Figure 7). The graft is de-aired by briefly releasing the hemostat clamp from the left-sided 8-mm side branch and then punching tiny holes through the graft with a small-gauge needle.
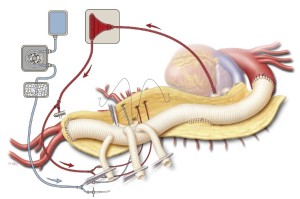
The right renal artery anastomosis is done next because of its medial location and because it is important to reestablish blood flow to one of the kidneys as soon as possible to reduce renal ischemic time. After the right renal artery perfusion catheter is removed, the right-sided 8-mm side branch is trimmed to the appropriate length and anastomosed in an end-to-end configuration with a continuous 5-0 polypropylene suture (Figure 7). After the graft is de-aired, the anastomosis is finished and the hemostat clamp released. Reinforcing mattress stitches are placed where needed with pledgeted 4-0 polypropylene suture.
Next, the more inferior of the two anteriorly-placed 10-mm branches is cut to the appropriate length and anastomosed in an end-to-end configuration to the SMA with a continuous 5-0 polypropylene suture (Figure 8). To facilitate this anastomosis, the selective perfusion catheter to the SMA is removed and selective flow to the celiac axis is discontinued; this enhances visualization of the anastomosis by preventing visceral back-bleeding through the SMA. Before the anastomosis is completed, the branch graft is briefly de-aired. Anastomotic bleeding is controlled with pledgeted 4-0 polypropylene mattress sutures.
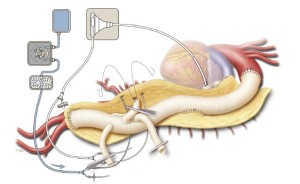
A hemostat clamp is then reapplied across the branch graft to the SMA to prevent back-bleeding from interfering with the celiac anastomosis. The celiac perfusion catheter is removed, and the uppermost 10-mm branch graft is trimmed to the appropriate length and then sewn to the origin of the celiac axis in an end-to-end configuration with a continuous 5-0 polypropylene suture (Figure 9). Before the celiac anastomosis is completed, the hemostat on the SMA graft is removed to facilitate the de-airing of the celiac graft. After the anastomosis is completed, the clamp on the celiac branch graft is released.
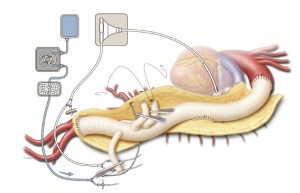
Often, the origin of the left renal artery is separated from the aortic wall as a button, and its proximal portion is mobilized. The remaining 8-mm side-branch graft is trimmed to the appropriate length; care is taken to ensure that the artery and the branch graft will not kink once the abdominal organs are returned to their anatomic positions. The renal perfusion catheter is removed, and like the anastomoses formed in the other bypassed vessels, the anastomosis is fashioned in an end-to-end configuration with a continuous 5-0 polypropylene suture (Figure 10). After the graft has been de-aired and the anastomosis completed, the clamp is removed. Protamine sulfate is administered to reverse the heparin. Indigo carmine is also administered intravenously to assess the adequacy of renal perfusion; ideally, blue dye should be visible in the urine within 20 minutes.
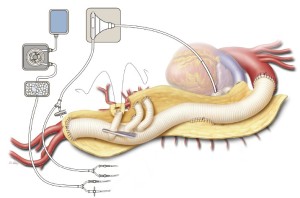
Hemostasis and closure
All anastomoses and suture ligatures are checked for bleeding and are reinforced as needed. The left lung is retracted posteriorly by using a moist laparotomy pad and a deep Deaver retractor. The atrial cannula is removed, and the purse string suture in the pulmonary vein is tied. This cannulation site closure is reinforced with a pledgeted 3-0 polypropylene suture. After protamine has been given and surgical hemostasis has been secured, blood products are transfused as necessary to reverse any coagulopathy. The cut edges of the opened native aorta are cauterized. The completed repair is inspected to make sure that the main graft and its branches lie properly without kinking (Figure 11). The left renal artery, proper hepatic artery, and intestinal arterial branches are palpated to check for pulses and ensure adequate blood flow. The kidneys are palpated for turgor, the bowel is visualized to confirm that it is well perfused, and the spleen is inspected to ensure that it has not been injured.
The field is irrigated with warm water to halt the cooling trend. A 19-French closed-suction abdominal drain is placed in the upper left retroperitoneal space. The left hemidiaphragm is reapproximated up to the costal margin with a continuous #1 polypropylene suture. The retractors are removed at this point. Two straight 36-French chest tubes are placed in an anteroapical and posterobasal position within the left chest cavity. Five #2 braided absorbable pericostal sutures are placed around the 6th and 7th ribs in a figure-of-eight fashion. Two #7 surgical steel wires are also placed around the ribs in a figure-of-eight fashion, one in the middle portion of the thoracotomy and one near the costal margin. The wires are twisted, cut, and buried, and the pericostal sutures are tied, which reapproximates the 6th and 7th ribs. The chest tubes are connected to a standard evacuation container that is connected to −20 cmH2O continuous suction. The abdominal fascia is closed with another continuous #1 polypropylene suture; this suture is carried to the left costal margin and tied to the suture from the diaphragm, which is tightened to ensure that there are no defects in the diaphragm closure. Both lungs are ventilated at this point. Two pericostal analgesia catheters are placed along the thoracotomy incision. The serratus anterior and latissimus dorsi muscles are reapproximated with separate #1 polypropylene sutures. The abdominal drain is connected to bulb suction. The wound is irrigated with antibiotic solution. The subdermal layer is reapproximated with continuous 2-0 absorbable monfilament suture, and the skin is closed with staples. Betadine ointment and then a sterile dressing are applied to the wound. Quarter-percent ropivacaine is administered via a timed-release reservoir connected to the pericostal analgesia catheters.
Comments
The use of the four-branched graft in TAAA repairs has been adopted by several groups (1-4). The potential disadvantages of using this approach are primarily related to the greater number of anastomoses involved in the fourbranch technique than in the traditional patch reattachment approach; collectively, the four branch anastomoses may take longer to complete than a single patch anastomosis, thus prolonging abdominal organ ischemic times. Additionally, to avoid postoperative organ ischemia due to graft kinking, the branch grafts need to be situated with proper length and orientation. Importantly, branch graft patency is 98% at 5 years, and the incidence of subsequent reoperation on the visceral segment of the aorta or its branches is essentially nil (4).
The abovementioned concerns appear to be offset by several key advantages that the branched graft technique provides (8,12,13). Most notably, it affords a durable repair in patients with connective tissue disease by minimizing the amount of residual native aorta in the visceral segment, thereby preventing the future development of patch aneurysm. The branched graft also provides an alternate repair option in patients whose visceral and renal arteries are far enough apart to make a patch reimplantation undesirable. In addition, the branched graft can be very useful in reoperations on patients who have developed a visceral patch aneurysm after previous TAAA surgery. Furthermore, by facilitating construction of the distal anastomosis before the visceral and renal anastomoses, using the branched graft enables earlier reperfusion of the iliac circulation, which provides important collateral blood flow to the spinal cord. The prefabricated branched graft also provides the advantage of flexibility, because it can be adapted to different anatomic needs. Additionally, the sequence of grafting can be varied according to the size, patency, and location of the visceral arteries. The surgeon can decide the order in which the anastomoses will be performed, allowing for earlier reperfusion of the kidneys and other organs as needed. Moreover, the technique allows for the creation of tension-free anastomoses, preventing early bleeding problems. Using the prefabricated graft further reduces the potential for bleeding by eliminating the anastomoses that would be necessary if separate grafts for the branch arteries were individually sutured to a standard aortic graft intraoperatively. Finally, in cases of visceral arterial occlusive disease, the stenotic ostial portion of the visceral branches can be addressed by transecting the vessel beyond the stenosis. This avoids the need for vessel endarterectomy or the placement of branch-vessel stents.
Acknowledgements
The authors thank Stephen N. Palmer, PhD, ELS, and Susan Y. Green, MPH, for editorial support. Figures used with the permission of Baylor College of Medicine.
Disclosure: Dr. Coselli serves as a consultant for Vascutek Ltd., a subsidiary of Terumo Corporation, and receives royalties related to Vascutek® Gelweave™ Coselli thoracoabdominal grafts.
References
- De Rango P, Estrera AL, Miller C 3rd, et al. Operative outcomes using a side-branched thoracoabdominal aortic graft (STAG) for thoraco-abdominal aortic repair. Eur J Vasc Endovasc Surg 2011;41:41-7.
- Kokotsakis J, Lazopoulos G, Ashrafian H, et al. Thoracoabdominal aneurysm repair using a four-branched thoracoabdominal graft: a case series. Cases J 2009;2:7144.
- Kouchoukos NT, Masetti P, Castner CF. Use of presewn multiple branched graft in thoracoabdominal aortic aneurysm repair. J Am Coll Surg 2005;201:646-9.
- Kulik A, Castner CF, Kouchoukos NT. Patency and durability of presewn multiple branched graft for thoracoabdominal aortic aneurysm repair. J Vasc Surg 2010;51:1367-72.
- Dardik A, Perler BA, Roseborough GS, et al. Aneurysmal expansion of the visceral patch after thoracoabdominal aortic replacement: an argument for limiting patch size? J Vasc Surg 2001;34:405-9; discussion 410.
- LeMaire SA, Carter SA, Volguina IV, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Ann Thorac Surg 2006;81:2063-78; discussion 2078.
- Schwill S, LeMaire SA, Green SY, et al. Endovascular repair of thoracic aortic pseudoaneurysms and patch aneurysms. J Vasc Surg 2010;52:1034-7.
- Bhamidipati CM, Coselli JS, LeMaire SA. Perfusion techniques for renal protection during thoracoabdominal aortic surgery. J Extra Corpor Technol 2012;44:P31-7.
- Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 1999;67:1931-4; discussion 1953-8.
- Coselli JS, LeMaire SA. Tips for successful outcomes for descending thoracic and thoracoabdominal aortic aneurysm procedures. Semin Vasc Surg 2008;21:13-20.
- Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9.
- Köksoy C, LeMaire SA, Curling PE, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg 2002;73:730-8.
- LeMaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:11-9; discussion 19.
- Vaughn SB, LeMaire SA, Collard CD. Case scenario: anesthetic considerations for thoracoabdominal aortic aneurysm repair. Anesthesiology 2011;115:1093-102.
- LeMaire SA, Jamison AL, Carter SA, et al. Deployment of balloon expandable stents during open repair of thoracoabdominal aortic aneurysms: a new strategy for managing renal and mesenteric artery lesions. Eur J Cardiothorac Surg 2004;26:599-607.





