Mycotic thoracoabdominal aneurysms
Introduction
Primary mycotic thoracoabdominal aortic aneurysms (MAAs) are a small subset of all aortic aneurysms, but left untreated are almost invariably fatal from rupture (1-3). Historically, the gold standard of treatment was wide surgical debridement and in-situ or extra-anatomical repair. Mortality rates of up to 40 per cent are associated with open surgical repair (1,4,5). This poor outcome may be as a result of the plethora of medical comorbidities, magnitude of surgical insult and presence of sepsis encountered in these patients.
The treatment of this life-limiting condition has been subject to evolution in line with the advent and acceptance of stent-graft technology and the development of open surgical adjuncts to stenting-hybrid surgery (6). Furthermore it has been suggested in the literature and is our belief that infection of the native aorta and super-infection of an aortic graft are differing disease entities and as such should be managed differently. This is reflected, perhaps anecdotally, in the re-infection rates reported in one series of endovascular repairs of native thoracic aortic infections vs. infected grafts [6.3% vs. 50%; P=0.08 (7)]. Mycotic aneurysms are defined by the presence of two or more of the following features: sepsis (fever, leucocytosis and pain), positive blood culture, positive culture from the aneurysmal wall, or characteristic radiological appearance (including irregular aortic wall, rapid growth rate, or saccular appearance of the aneurysm). Negative blood cultures and absence of pyrexia do not exclude the diagnosis when the patient has presented with signs of infection and had characteristic radiological findings but had already been commenced on antibiotics.
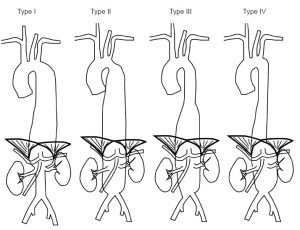
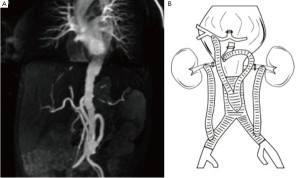
We will discuss in turn the pitfalls and hazards of modern treatment of primary MAAs, according to anatomical location in line with the Crawford classification (8). There has been lack of clarity in reporting outcomes from true descending thoracic aneurysms (DTAs) in which the proximal and distal stent landing zones do not impinge upon the origin of arch and/or visceral arteries, and type I thoracoabdominal aneurisms (TAAAs) (9). We will discuss descending thoracic aneurysms as a separate entity to true type I TAAA as outcomes will patently be very different. We believe that clarity in description of these aneurysms treated is essential in the evolution of our knowledge in how MAAs are best managed (Figure 1).
Descending thoracic aneurysms
The advent of thoracic endovascular aneurysm repair (TEVAR) graft technology has transformed the management of mycotic aneurysms.
Semba et al. were the first to describe endovascular repair of three mycotic DTAs in combination with antibiotic therapy. They reported no perioperative mortality and no complications from persistent bacteraemia at median follow-up of 24 months (10).TEVAR has reduced the 28% (11) mortality associated with open repair to as low as 11% to 15% (12,13). Some medium-term follow-up data post-TEVAR for MAA reports patients alive as long as 83 months with a survival of 73% at median follow-up of 20 months (13).
Complications reported to be associated with TEVAR for mycotic DTAs include perioperative rupture, stent migration, and malposition with a type I endoleak (14) (Figure 2).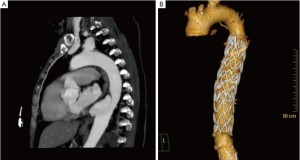
Kan et al. performed a systematic review of endovascular treatment for mycotic aortic aneurysms. They included 16 abdominal and 32 thoracic aneurysms, of which only one was ostensibly thoracoabdominal (15).
The 30-day mortality, due to sepsis or massive bleeding, was 10.4% (five patients). There were five late mortalities (10.4%), two died of cardiac disease, and three died with graft-related bleeding problems. The 12-month actual survival rate of the healed group was 94.0±4.0%, and that of the persistently infected group was 39.0±17.0%. There was no significant influence of aneurysm location.
Interestingly, age ≥65 years, rupture of the aneurysm (including aortoenteric fistula and aortobronchial fistulae), and fever at the time of surgery were identified as significant predictors of persistent systemic infection, defined as fever, signs of sepsis, or haemorrhage. Pre-operative use of antibiotics for longer than one week and an adjunct procedure combined with EVAR were identified as significant protective factors for persistent infection. However, by multivariate logistic regression analysis, the only significant independent predictors of persistent systemic infection identified were rupture of aneurysm and fever.
In our reported series of four ruptured and one intact mycotic DTA, TEVAR was associated with one perioperative death and one type II endoleak, which spontaneously resolved, over a mean follow-up period of 30.5 months (6). Despite the theoretical risks of infection of the stent graft, the surgical insult associated with explanting a TEVAR and performing open surgery once sepsis has subsided renders this impossible. TEVAR for mycotic DTA should be considered the definitive procedure rather than an adjunct to deferred open surgery.
Ortner’s syndrome - recognition and management
An unusual complication of TEVAR is compression of the left recurrent laryngeal nerve as it hooks around the arch of the aorta (16-19). The most sensitive region of that of the ductus arteriosus and aneurysm of a patent ductus arteriosus has been described as a cause of Orter’s syndrome (20,21). The left vocal cord will adopt a paramedian position and cause hoarseness of voice. This is can be due to an aneurysm itself (22) or acute sac expansion following sac thrombosis subsequent to stent deployment (23). In one reported case, the hoarseness of voice actually improved following endovascular treatment of a saccular aneurysm (24). There is no particular strategy to obviate from acute sac expansion post-stenting; however it is important to be cognizant of this potential problem and aware that the condition is likely to improve with conservative management.
Type I, II and III TAAA
Open repair for mycotic type I, II and III TAAAs has always been historically associated with mortality of up to 40% (25-27) due to the magnitude of the procedure in unwell, septic patients. Endovascular technology offers a less invasive method of dealing with the mycotic aneurysm.
Initially, treatment for true type I MAA was only practicably possible by thoracic stenting with retrograde revascularization or coverage of the coeliac artery in order to extend the distal landing zone (Figure 3). Intentional coeliac artery coverage with preoperative assessment of coeliac to SMA anastomoses for complex TAAA has been shown to be associated with relatively low rates of mortality and morbidity (28-31).
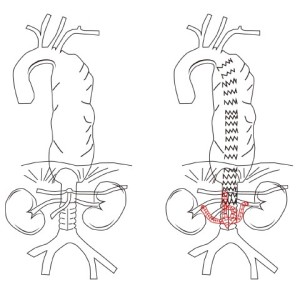
More recently, development of endovascular stent technology have potentially allowed for repair of type I MAAs in patients with favourable anatomy using thoracic stents with a customized distal scallop for the coeliac axis (32). No reports have been forthcoming with respect to MAAs, however this may be a viable option in patients with relatively ‘stable’ MAAs who can tolerate the time necessary for stent graft manufacture and delivery.
In type II MAA, there are two practical options for repair in the systemically unwell patient: totally endovascular or hybrid repair.
For type III MAA, we would advocate stratifying management along the lines of fitness for surgery. In patients who are deemed unfit for the open type III approach to repair, we advocate hybrid surgery; endovascular stenting with either retrograde or antegrade visceral revascularization from the lower aorta/iliac arteries or the ascending/descending aorta, respectively.
In relatively fit patients without shock, an open approach has the benefit of allowing for extensive debridement of aorta and peri-aortic tissue.
Systemically unwell/unfit patients
Totally endovascular repair
In one series of nine mycotic aneurysms and pseudoaneurysms (five thoracic, four paravisceral) undergoing open repair, four required visceral revascularization. The authors report no hospital death, limb loss, renal failure, or intestinal ischemia; two late deaths occurred due to sepsis and pneumonia at three months and 77 months. Eight patients were alive after a mean follow-up of 36 months and no late graft infection was evident (33). Clearly the numbers are small and these promising results do not correspond to most published experiences.
Totally endovascular repair has been described, involving embolization of involved visceral arteries or branched grafts. Although embolisation of the coeliac axis in MAA has been described, prior to any such visceral artery embolization selective mesenteric angiography to confirm patent collateral supply is advocated. This single case report utilized a custom made Powerlink (Endologix, Irvine, CA, USA) abdominal endoluminal graft cuff measuring 28 mm × 5.5 cm. Deployment was to the superior mesenteric artery orifice, which was prophylactically stented to prevent coverage in the case of stent migration (34). Good appearances were seen on computed tomography at one-year follow-up.
Hybrid repair
For the majority of patients presenting with mycotic type II and III TAAAs there is insufficient time to wait for custom made endovascular branched grafts. To avoid the necessity of a totally surgical repair, a hybrid open/endovascular approach has been adopted in some centers. The hybrid repair for type II or III MAA would normally entail aortic visceral artery debranching with retrograde visceral revascularization and stenting of the aneurysmal thoraco-abdominal segment (35).
The procedure is performed through a midline laparotomy incision. The key decision making aspect of the procedure tailored to each individual patient is the location of the distal landing zone and consequent ‘take-off’ of the retrograde revascularization grafts - the distal aorta, common iliac arteries or even proximal external iliac arteries (if the aneurysmal process extends down into the common iliac arteries) have all been used. Subsequent steps include graft construction by anastomosing conduits of the desired caliber and conformation, deployment of the endovascular stent-graft and closure of the retroperitoneum.
While one team performs the laparatomy and exposure of the visceral and renal arteries and the take-off vessels (distal aorta, iliac arteries), another person can construct the retrograde grafts.This configuration varies and is often only decided on during the operation. Generally a Dacron bifurcation 14 mm × 7 mm graft is used with 6- to 8-mm grafts sewn on to the graft for the renal arteries. If necessary, a 10-mm conduit is used to deliver the stent-grafts. If both common iliacs are used for the take-off point, two bifurcated grafts can be used (Figure 4).
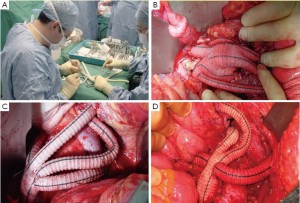
Similar to endovascular repair of type I MAA, stenting of the arch as part of the hybrid repair carries the risk of acute sac expansion post-aneurysm exclusion. This may exacerbate what pre-operatively may have been actual or potential bronchial or oesophageal compression.
Paraplegia risk
A potentially devastating consequence of hybrid surgery for type I, II and III TAAA is spinal cord ischaemia and subsequent paraplegia. We have previously reported an analysis of stenting procedures at Saint Mary’s Hospital. Here we found that a minimum coverage of 55% of the aorta measured from the brachiocephalic origin is associated with paraplegia (36). Patients undergoing fenestrated or branched endovascular grafting had a spinal cord ischaemia rate of 2/14 (14%), and a paraplegia rate of 1/14 (7%), whereas patients undergoing the visceral hybrid operation had a spinal cord ischaemia rate of 16/80 (20%) and a paraplegia rate of 9/80 (11%). Spinal cord ischaemia also has a high incidence in open thoracoabdominal surgery, at between 5 and 20% (37,38).
The incidence of spinal cord ischaemia in open thoracoabdominal surgery has been shown to be reduced by the use of spinal cerebrospinal fluid (CSF) drainage (39). Our practice is to use selective CSF drainage in cases deemed to have a high risk of spinal cord ischaemia. Other spinal cord protection strategies which have been advocated include blood pressure control and intercostal artery re-implantation (40). The additive protection imparted by intercostal re-implantation over and above other strategies suggests that the hybrid approach may have theoretical advantages over a totally endovascular approach if this strategy is employed.
Systemically well/fit patients with type III MAA
Systemically well patients who are deemed fit may be considered for open type III repair. The advantage of this approach in patients who are able to tolerate the surgery is that aortic debridement can be undertaken as well as direct tissue biopsy for microbiological culture.
The operative approach is via a thoracolaparotomy (Figure 6). The incision begins in the sixth intercostal space, and crosses the costal margin and upper rectus abdominis to end in the abdominal midline. We dissect the muscle from the sixth rib using diathermy and periosteal elevator and excise this rib. For more proximal disease, a higher thoracic incision is needed. Once a segment of the costal margin is excised, we find it allows for good muscular apposition to the thoracic closure without the need for insertion of sutures into adjacent intercostal spaces.
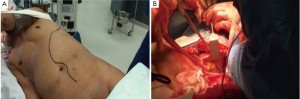
The essence of the open type II and III TAAA repair involves a ‘circular’ anastomosis of the graft to the descending thoracic aorta with visceral re-implantation using the Carrel patch technique.
Type IV TAAAs
Dubois et al. have reported on 12 patients in a wider series who had type IV MAA (41). These were reconstructed by aorto-aortic interposition with re-implantation or bypass to visceral vessels. The approach seems to have been mostly retroperitoneal with involvement of the visceral segment.
We advocate a modified approach to the type IV repair for sepsis. This involves a thoracolaparotomy akin to the approach for open type III repair, rather than our routine rooftop incision (Figure 6). The thoracolaparotomy allows for rapid and safe proximal control in this setting which is particularly important in dissecting a potentially friable MAA.
Mycotic aneurysm involving fistulation with the aerodigestive tract
The previously mentioned systematic review of endovascular treatment of MAA by Kan et al. reported that the occurrence of aorto-enteric and aorto-bronchial fistula is significantly related to persistent infection (15).
A later study reported that mortality was also almost double in MAA associated with fistula as opposed to without (13). Also in line with the review by Kan et al., this series suggests that preoperative antibiotics for three days or more is protective against long term infection.
A single centre experience reports on seven aortobronchial fistulas, 2.7% of their five year thoracic stenting practice. These patients presented with haemoptysis. No standard post-operative antibiotic regimen was followed. There were no endoleaks, no incidence of paraplegia, and no endoluminal graft infections in this somewhat heterogeneous group including those with an infected previously-placed stent-graft and an infected open repair. Survival was 100% at both 30 days and one year. This group concluded that endovascular management of aortobronchial fistulas appears to be safe and well tolerated, with minimal risk of prosthesis infection (42). This has not been our experience. In a small series of seven aortobronchial fistulas over the last decade we have had no survivors beyond two years.
Pre- and post-operative antibiotic therapy
In as many as 25% of cases, no bacteriological species is identified. This is thought to be due to perioperative antibiotic therapy and lack of direct biopsy in the endovascular age. When identified, gram-negative species are identified in as many as 50% of cases. Of these, Salmonella is most common (41).
Cina et al. suggest that gram-negative microorganisms are found in 47% of mycotic TAAAs and that there is a trend toward increased mortality for these organisms. They advocate the intravenous use of two synergistic antibiotics, particularly in gram-negative infections because of the invasive potential of these microorganisms and the associated poor prognosis. They further suggest no difference in survival or recurrence rate between series advocating lifelong therapy and those suggesting prolonged (six weeks to 12 months) therapy (43). This paradigm may have to be altered in the evolving era of endovascular therapy of MAA. We have reported positive blood cultures in 58% (11/19), with an identifiable source for the infection in seven patients, including spinal osteomyelitis, urosepsis, chest sepsis and a psoas abscess.
Stanley et al., have reported antibiotic soaking of a stent graft prior to implanting for MAA (44). Their rational was that rifampicin bonding to Dacron grafts has been shown to decrease the reinfection rate when treating graft infection with in situ replacement (45,46). Although no evidence base is likely to be forthcoming, the practice seems reasonable. Similar anecdotal evidence has been reported for rifampicin-bonded gelatin-impregnated Dacron grafts in two patients with mycotic aneurysms infected by Staphylococcus aureus (47). In our unit we use a six-week regime of intravenous antibiotics, guided by culture results, or broad-spectrum empirically-chosen antibiotics.In summary, we agree with the views of Smith and Taylor, who recommend the establishment of an international registry to establish best practice in this rare disease entity (48).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Müller BT, Wegener OR, Grabitz K, et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg 2001;33:106-13.
- Reddy DJ, Lee RE, Oh HK. Suprarenal mycotic aortic aneurysm: surgical management and follow-up. J Vasc Surg 1986;3:917-20.
- Gross C, Harringer W, Mair R, et al. Mycotic aneurysms of the thoracic aorta. Eur J Cardiothorac Surg 1994;8:135-8.
- Fillmore AJ, Valentine RJ. Surgical mortality in patients with infected aortic aneurysms. J Am Coll Surg 2003;196:435-41.
- Moneta GL, Taylor LM Jr, Yeager RA, et al. Surgical treatment of infected aortic aneurysm. Am J Surg 1998;175:396-9.
- Vallejo N, Picardo NE, Bourke P, et al. The changing management of primary mycotic aortic aneurysms. J Vasc Surg 2011;54:334-40.
- Patel HJ, Williams DM, Upchurch GR Jr, et al. Late outcomes of endovascular aortic repair for the infected thoracic aorta. Ann Thorac Surg 2009;87:1366-71; discussion 1371-2.
- Crawford ES, Coselli JS. Thoracoabdominal aneurysm surgery. Semin Thorac Cardiovasc Surg 1991;3:300-22.
- Modarai B, Bell R, Taylor P. Letter by Modarai et al regarding article, “Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair”. Circulation 2009;120:e89.
- Semba CP, Sakai T, Slonim SM, et al. Mycotic aneurysms of the thoracic aorta: repair with use of endovascular stentgrafts. J Vasc Interv Radiol 1998;9:33-40.
- Hsu RB, Lin FY. Infected aneurysm of the thoracic aorta. J Vasc Surg 2008;47:270-6.
- Patel HJ, Williams DM, Upchurch GR Jr, et al. Late outcomes of endovascular aortic repair for the infected thoracic aorta. Ann Thorac Surg 2009;87:1366-71; discussion 1371-2.
- Clough RE, Black SA, Lyons OT, et al. Is endovascular repair of mycotic aortic aneurysms a durable treatment option? Eur J Vasc Endovasc Surg 2009;37:407-12.
- Jones KG, Bell RE, Sabharwal T, et al. Treatment of mycotic aortic aneurysms with endoluminal grafts. Eur J Vasc Endovasc Surg 2005;29:139-44.
- Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg 2007;46:906-12.
- Bickle IC, Kelly BE, Brooker DS. Ortner’s syndrome: a radiological diagnosis. Ulster Med J 2002;71:55-6.
- Gulel O, Elmali M, Demir S, et al. Ortner’s syndrome associated with aortic arch aneurysm. Clin Res Cardiol 2007;96:49-50.
- Gupta P, Sharma S. Ortner’s syndrome secondary to aortic aneurysm. Ann Acad Med Singapore 2012;41:40-1.
- Kopp R, Linn J, Stelter K, et al. Hybrid operation for a distal aortic arch aneurysm causing left recurrent nerve palsy - Ortner’s syndrome. Laryngorhinootologie 2008;87:723-7.
- Day JR, Walesby RK. A spontaneous ductal aneurysm presenting with left recurrent laryngeal nerve palsy. Ann Thorac Surg 2001;72:608-9.
- Kokotsakis J, Misthos P, Athanassiou T, et al. Acute Ortner’s syndrome arising from ductus arteriosus aneurysm. Tex Heart Inst J 2008;35:216-7.
- Daou M, Moser D, Bentz MH. Ortner’s syndrome and giant-cell vasculitis. Rev Med Interne 2006;27:889-91.
- Escribano JF, Carnés J, Crespo MA, et al. Ortner’s syndrome and endoluminal treatment of a thoracic aortic aneurysm: a case report. Vasc Endovascular Surg 2006;40:75-8.
- Stoob K, Alkadhi H, Lachat M, et al. Resolution of hoarseness after endovascular repair of thoracic aortic aneurysm: a case of Ortner’s syndrome. Ann Otol Rhinol Laryngol 2004;113:43-5.
- Müller BT, Wegener OR, Grabitz K, et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg 2001;33:106-13.
- Fillmore AJ, Valentine RJ. Surgical mortality in patients with infected aortic aneurysms. J Am Coll Surg 2003;196:435-41.
- Moneta GL, Taylor LM Jr, Yeager RA, et al. Surgical treatment of infected aortic aneurysm. Am J Surg 1998;175:396-9.
- Mehta M, Darling RC 3rd, Taggert JB, et al. Outcomes of planned celiac artery coverage during TEVAR. J Vasc Surg 2010;52:1153-8.
- Leon LR Jr, Mills JL Sr, Jordan W, et al. The risks of celiac artery coverage during endoluminal repair of thoracic and thoracoabdominal aortic aneurysms. Vasc Endovascular Surg 2009;43:51-60.
- Belenky A, Haddad M, Idov I, et al. Celiac trunk embolization, as a means of elongating short distal descending thoracic aortic aneurysm necks, prior to endovascular aortic repair. Cardiovasc Intervent Radiol 2009;32:923-7.
- Vaddineni SK, Taylor SM, Patterson MA, et al. Outcome after celiac artery coverage during endovascular thoracic aortic aneurysm repair: preliminary results. J Vasc Surg 2007;45:467-71.
- Da Rocha M, Riambau VA. Experience with a scalloped thoracic stent graft: a good alternative to preserve flow to the celiac and superior mesenteric arteries and to improve distal fixation and sealing. Vascular 2010;18:154-60; discussion 161.
- Ting AC, Cheng SW, Ho P, et al. Surgical treatment of infected aneurysms and pseudoaneurysms of the thoracic and abdominal aorta. Am J Surg 2005;189:150-4.
- Kpodonu J, Ramaiah VG, Wheatley GH 3rd, et al. Customized endoluminal graft to treat a suspected saccular thoracoabdominal mycotic aneurysm. Ann Thorac Surg 2008;85:1463-5.
- Rimmer J, Wolfe JH. Type III thoracoabdominal aortic aneurysm repair: a combined surgical and endovascular approach. Eur J Vasc Endovasc Surg 2003;26:677-9.
- Drinkwater SL, Goebells A, Haydar A, et al. The incidence of spinal cord ischaemia following thoracic and thoracoabdominal aortic endovascular intervention. Eur J Vasc Endovasc Surg 2010;40:729-35.
- Coselli JS, LeMaire SA, Miller CC 3rd, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg 2000;69:409-14.
- Safi HJ, Hess KR, Randel M, et al. Cerebrospinal fluid drainage and distal aortic perfusion: reducing neurologic complications in repair of thoracoabdominal aortic aneurysm types I and II. J Vasc Surg 1996;23:223-8; discussion 229.
- Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9.
- Acher CW, Wynn MM, Mell MW, et al. A quantitative assessment of the impact of intercostal artery reimplantation on paralysis risk in thoracoabdominal aortic aneurysm repair. Ann Surg 2008;248:529-40.
- Dubois M, Daenens K, Houthoofd S, et al. Treatment of mycotic aneurysms with involvement of the abdominal aorta: single-centre experience in 44 consecutive cases. Eur J Vasc Endovasc Surg 2010;40:450-6.
- Wheatley GH 3rd, Nunez A, Preventza O, et al. Have we gone too far? Endovascular stent-graft repair of aortobronchial fistulas. J Thorac Cardiovasc Surg 2007;133:1277-85.
- Cinà CS, Arena GO, Fiture AO, et al. Ruptured mycotic thoracoabdominal aortic aneurysms: a report of three cases and a systematic review. J Vasc Surg 2001;33:861-7.
- Stanley BM, Semmens JB, Lawrence-Brown MM, et al. Endoluminal repair of mycotic thoracic aneurysms. J Endovasc Ther 2003;10:511-5.
- Goëau-Brissonnière O, Mercier F, Nicolas MH, et al. Treatment of vascular graft infection by in situ replacement with a rifampin-bonded gelatin-sealed Dacron graft. J Vasc Surg 1994;19:739-41.
- Lachapelle K, Graham AM, Symes JF. Antibacterial activity, antibiotic retention, and infection resistance of a rifampin-impregnated gelatin-sealed Dacron graft. J Vasc Surg 1994;19:675-82.
- Gupta AK, Bandyk DF, Johnson BL. In situ repair of mycotic abdominal aortic aneurysms with rifampin-bonded gelatin-impregnated Dacron grafts: a preliminary case report. J Vasc Surg 1996;24:472-6.
- Smith JJ, Taylor PR. Endovascular treatment of mycotic aneurysms of the thoracic and abdominal aorta: the need for level I evidence. Eur J Vasc Endovasc Surg 2004;27:569-70.