Leaflet extension for repairing rheumatic mitral valve regurgitation
Introduction
It has been established that mitral valve (MV) repair is preferred over replacement in patients with mitral regurgitation (MR) caused by degenerative disease. In contrast, valve reconstruction for rheumatic MR remains controversial. Type IIIa MR due to rheumatic leaflet restriction often renders valve repair challenging and may predict a less successful repair. However, the utilization of leaflet mobilization and extension with autologous pericardium in order to increase leaflet area and surface of coaptation may achieve satisfactory results (1-3). This article represents our single-center experience of leaflet extension in rheumatic MR, with emphasis on description of the technique including tips on safeguards and pitfalls.
Safeguards and pitfalls
Indications for leaflet extension
Repair techniques for type IIIa rheumatic MR are based on Carpentier reconstruction principles (4), with some modifications tailored to the individual patient. Commissurotomy, papillary muscle splitting, excision of shortened chordae and thinning of leaflets are initially performed to improve mobility and pliability of the leaflets. When leaflet and subvalvular mobilization are deemed inadequate to compensate for extensive tissue retraction and leaflet hypoplasia, leaflet extension or augmentation is adopted to increase the surface area of the leaflet, providing increased mobility and surface for leaflet coaptation.
Anterior leaflet extension is recommended when the area of the leaflet is smaller than the 26 mm annuloplasty sizer, which is the smallest adult prosthetic ring (Figure 1A). Alternatively, leaflet augmentation is also undertaken when the vertical height of the anterior leaflet is less than 26 mm, as this has been associated with failure of repair (Figure 1B) (5). Posterior leaflet extension is undertaken when there is severe leaflet retraction, especially when the vertical height is less than 10 mm. It is important to recognize that retraction of the posterior leaflet is frequently present in rheumatic disease. If overlooked or misunderstood, this lesion may be misinterpreted by echo or at surgery as prolapse of the anterior leaflet where in actual fact it is a pseudo-prolapse of the anterior leaflet relative to a retracted-restricted posterior leaflet.
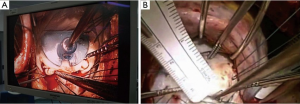
Which leaflet to extend?
It remains somewhat debatable whether the anterior or the posterior leaflet should be extended in restrictive rheumatic disease, with equal numbers of publications to support either approach. However, the most frequent mechanism in rheumatic MR, as reported by Carpentier, is retraction of the posterior leaflet due to progressive fibrosis of the leaflet and subvalvular apparatus (4). Therefore in most cases the anterior leaflet is not retracted, and we generally do not extend the anterior leaflet but focus instead on the posterior leaflet. In our experience, the proportion of patients having posterior, anterior and both leaflet(s) extensions were 75%, 15% and 10% respectively (3).
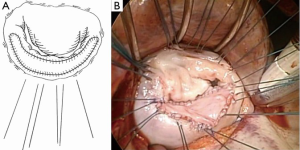
Size of the leaflet extension patch
The patch is usually ovoid in shape, designed to create a curtain-like posterior leaflet to allow for free and generous coaptation against the anterior leaflet (Figure 2). The reconstructed posterior leaflet is commonly about 15-20 mm in height and spans from commissure to commissure in width. A conscious effort is made to avoid excessive height of the extended posterior leaflet (not more than 20 mm) and use of an undersized prosthetic ring to prevent systolic anterior motion (SAM) causing left ventricular outflow tract obstruction. We have not encountered SAM following rheumatic MV repair in our practice nor reported in the literature. This is probably due to the fact that the rheumatic leaflets are rather fibrotic, short and stiff, thus not displaying the propensity of the bulky myxomatous leaflets implicated in SAM. For the anterior leaflet, an incision is made 2 mm from the annulus and extended to both commissures. Two alternative methods of sizing of the patch may be used. Firstly, using nerve hooks to apply traction on the primary chords, pulling down against the posterior leaflet and the defect itself creates the size of the patch. Secondly, with the use of a ring annuloplasty sizer, the patch is sized over a 28-30 mm template. Regardless of whether it is the anterior or the posterior leaflet that is extended, it is important for the width of the pericardial patch to be of generous size, covering the defect created from commissure to commissure. This is to ensure complete mobilization of the entire restricted leaflet, an enlarged leaflet surface area and sufficient surface of coaptation between the opposing leaflet edges.
Sizing of annuloplasty ring after leaflet extension
Leaflet extensions allow for placement of a larger annuloplasty ring, thereby reducing the risk of stenosis, especially important in rheumatic disease patients (2-4). The size of the ring is determined by the final dimension/area of the anterior leaflet. Thus, if the anterior leaflet was extended, then naturally a larger size ring was implanted. Following posterior leaflet extension, one could size the entire perimeter of the annulus instead of the anterior leaflet, allowing for the possibility of oversizing (usually one size) of the prosthetic ring.
Sutures and suturing technique
It is advisable to first apply annuloplasty sutures around the posterior circumference of the mitral annulus. This initial step improves valve exposure for accurate analysis and avoids pericardial patch dehiscence due to needle-hole perforation, than if the larger annuloplasty sutures were to be inserted after the patch had been sewn into place. The patch is sutured in using two continuous 5/0 non-absorbable monofilament sutures (polypropylene) with fixations at three or four points to prevent purse-string effect. The patch is oriented so that the smooth surface of the pericardium faces the left atrial side to reduce the potential for thrombogenesis.
Patch material for leaflet extension
Chauvaud first described the use of autologous pericardium two decades ago (1). It is readily available, easy to handle and its pliability make it an obvious choice to correct leaflet defects. When compared to commercial bovine pericardium, autologous pericardium is non-antigenic, avoids the risk of xenograft viral transmission and does not add to cost. The autologous pericardium is treated with 0.6% glutaraldehyde-buffered solution for 5 to 10 minutes. The glutaraldehyde solution makes the pericardium stiffer, rendering it easier to handle. Adherence to the 5-10 minutes’ duration of glutaraldehyde pre-treatment is important as untreated pericardium suffers from acute tissue shrinkage and contracture, whereas prolonged treatment could cause late fibrosis and excessive calcification. Finally, there is recent histologic evidence that autologous pericardium is superior over a new patch material, porcine intestinal submucosa extracellular matrix (CorMatrix), for valve reconstruction, demonstrating more tissue infiltration, remodeling, vascularization and neointima formation with autologous pericardium (6).
Comments
Leaflet extension is feasible for rheumatic MR and complements the armamentarium of Carpentier’s valve reconstruction methods. The technique is reproducible and offers encouraging midterm outcomes (1,3). Longer follow up will establish the potential durability of this technique. Wider utilization of this technique may increase the success of repair in complex rheumatic MV disease.
Acknowledgements
Funding: This study was supported by the National Heart Institute (Institut Jantung Negara), Kuala Lumpur, Malaysia.
Disclosure: The authors declare no conflict of interest.
References
- Chauvaud S, Jebara V, Chachques JC, et al. Valve extension with glutaraldehyde-preserved autologous pericardium. Results in mitral valve repair. J Thorac Cardiovasc Surg 1991;102:171-7; discussion 177-8. [PubMed]
- Chauvaud S, Fuzellier JF, Berrebi A, et al. Long-term (29 years) results of reconstructive surgery in rheumatic mitral valve insufficiency. Circulation 2001;104:I12-5. [PubMed]
- Dillon J, Yakub MA, Nordin MN, et al. Leaflet extension in rheumatic mitral valve reconstruction. Eur J Cardiothorac Surg 2013;44:682-9. [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Gupta A, Gharde P, Kumar AS. Anterior mitral leaflet length: predictor for mitral valve repair in a rheumatic population. Ann Thorac Surg 2010;90:1930-3. [PubMed]
- Zaidi AH, Nathan M, Emani S, et al. Preliminary experience with porcine intestinal submucosa (CorMatrix) for valve reconstruction in congenital heart disease: histologic evaluation of explanted valves. J Thorac Cardiovasc Surg 2014;148:2216-4, 2225.e1.





