Progressive design concepts in off-pump left ventricular remodeling mitral valve repair devices
Functional mitral regurgitation (FMR) occurs in the myopathic ventricle when papillary muscle displacement with leaflet tethering and/or annular dilatation causes valvular insufficiency. The resultant left ventricular (LV) volume overload then potentiates further negative remodeling. Current surgical management of FMR involves either mitral valve repair (MVr) or mitral valve replacement (MVR), each of which are associated with significant perioperative risk and poor late outcomes. This has led to the development of novel methods of reshaping the left ventricle and mitral valve to treat FMR while minimizing the operative risk and preventing negative ventricular remodeling. While off-pump devices for mitral leaflet repair including percutaneous mitral clips, trans-apical neochordae and percutaneous mitral replacement devices are under investigation, this report reviews the current status of both passive and active off-pump ventricular and mitral annular reshaping devices.
Passive reshaping
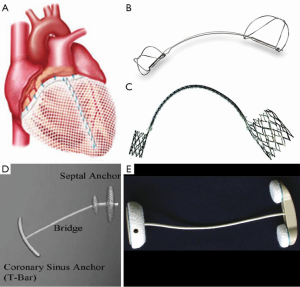
The initial attempt to treat global LV dysfunction in the setting of mitral regurgitation (MR) was the addition of the CorCap Cardiac Support Device (CSD) (Acorn Cardiovascular Inc., St. Paul, MN, USA) at the time of standard mitral valve surgery. The CSD is a fabric mesh implanted around the heart to provide circumferential diastolic support and limit negative LV remodeling by reducing ventricular wall stress (Figure 1A). The mitral valve stratum of the Acorn Trial (1) prospectively randomized patients with non-ischemic dilated cardiomyopathy, ≥2+ MR and NYHA class II-IV heart failure to MVR/r alone (n=102) or MVR/r + CSD (n=91). At five-year follow-up there was an overall mortality of 30% and an overall freedom from recurrent MR or reoperation of 81%. There were no significant differences in survival, cardiac transplant, use of assist devices or biventricular pacemakers, or adverse events between the two groups. However, there was a greater reduction in LV end-diastolic volume (mean difference 16.5 mL, P=0.05) and an improvement in sphericity index (mean difference 0.071 units, P=0.002) in the CSD group, suggesting an additive effect of CSD and mitral valve surgery on reverse LV remodeling.
Active reshaping
Stand-alone device reshaping therapy for FMR has two general approaches: annular and ventricular. The following are examples of device strategies.
Coronary sinus (CS) annular reshaping
CS mitral valve annuloplasty devices are inserted percutaneously into the CS to constrict the mitral annulus and improve leaflet coaptation without the need for open surgery or cardiopulmonary bypass. The TITAN trial (2) compared outcomes of patients who received the Carillon Mitral Contour System (Cardiac Dimensions, Kirkland, WA, USA; Figure 1B) (n=36) to those from whom the device was acutely removed due to coronary artery compromise or lack of FMR reduction (n=17). The investigators demonstrated a decrease in LV dimensions, mitral annular dimensions, and FMR (80% reduction of 1 or more grades), as well as improvements in 6-minute walk distance (6MWD), quality of life metrics and NYHA functional class at two years. One year mortality was similar between the two groups (22.2% vs. 23.5%). The EVOLUTION I trial (3) evaluated safety and efficacy of the MONARC device (Edwards Lifesciences, Irvine, CA, USA; Figure 1C) in 72 FMR patients. Device implantation was successful in 82% with a 12-month survival of 87%. The investigators found a mean decrease in FMR grade from 2.5 to 1.8 (P=0.002) and a decrease in 1 or more grades in 50% of patients. They also noted decreases in LV dimensions and FMR indices, as well as improvements in LV ejection fraction and NYHA functional class. Both studies identified coronary artery compromise as a complication of CS device implantation.
Direct mitral annular reshaping devices
The Mitralign Bident system (Mitralign, Inc., Tewksbury, MA, USA) places sutured pledgets directly into the posterior annulus via a catheter across the aortic valve, plicating them together to achieve reduction annuloplasty. Use of the system has been successfully reported in one patient with FMR and class III heart failure with a resultant decrease in proximal isovelocity surface area radius (0.7 to 0.4 cm), effective regurgitant orifice area (0.3 to 0.1 cm2) and mitral regurgitant volume (49 to 10 mL). Enrollment was recently completed in a European clinical trial (4).
Percutaneous septal sinus shortening
The Percutaneous Septal Sinus Shortening device (PS3 System; MVRx, Inc., Belmont, CA, USA; Figure 1D) anchors a cord between the CS and the atrial septum which is shortened to reduce mitral annular septolateral distance. Following an initial safety and feasibility study in humans (5), the MAVERIC phase I clinical trial (n=11) was conducted with initial results reported at Transcatheter Cardiovascular Therapeutics 2014. One patient required drainage of a pericardial effusion and one required mitral valve operation following device dislocation. At thirty days there was a decrease in mean FMR grade (3.4 to 1.5, P<0.01), regurgitant volume (P<0.01), septolateral diameter (P<0.01), pulmonary artery pressure (P=0.05) and NYHA class (P=0.02).
Combined mitral annular and ventricular reshaping
The Coapsys annuloplasty system (Myocor, Inc., Maple Grove, MN, USA; Figure 1E) consists of two epicardial pads connected by a cord, which is implanted under echocardiographic guidance and compresses the LV at both the level of the mitral annulus and papillary muscles. This aims to improve mitral leaflet coaptation and reduce LV dimensions and wall stress. The RESTOR-MV trial (6) randomized patients with ischemic dilated cardiomyopathy and ≥2+ FMR undergoing CABG to either CABG/MVr (n=75) or CABG/Coapsys (n=74). The study was terminated after the sponsor failed to secure ongoing funding. At two years, the Coapsys patients had an improved overall survival (87% vs. 77%, P=0.038) and a greater freedom from adverse events (76% vs. 63%, P=0.022). The Coapsys group had a smaller reduction in FMR grade than the MVr group (decrease by ≥2 grades: 66.7% vs. 92.0%, P=0.02) but a greater improvement in LV end-diastolic dimension (P=0.02). Both groups demonstrated similarly improved NYHA functional class (P=0.86). Four-year midterm follow-up of RESTOR-MV patients at a single randomization center (n=31) suggested a persistent survival advantage of Coapsys over MVr (74% vs. 50%, P=0.09). These data demonstrate a beneficial clinical effect of reshaping both the mitral valve and the LV in FMR.
Conclusions
Off-pump LV remodeling MVr devices may provide improvement in both FMR and LV dysfunction with significant clinical benefit shown in early trials. These approaches may eventually offer clinical advantage over traditional mitral valve surgery for FMR.
Acknowledgements
Disclosure: Dr. Grossi has development agreements with Edwards Lifesciences but none associated with the technology discussed in this manuscript. The authors declare no conflict of interest.
References
- Acker MA, Jessup M, Bolling SF, et al. Mitral valve repair in heart failure: five-year follow-up from the mitral valve replacement stratum of the Acorn randomized trial. J Thorac Cardiovasc Surg 2011;142:569-74, 574.e1.
- Siminiak T, Wu JC, Haude M, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail 2012;14:931-8. [PubMed]
- Harnek J, Webb JG, Kuck KH, et al. Transcatheter implantation of the MONARC coronary sinus device for mitral regurgitation: 1-year results from the EVOLUTION phase I study (Clinical Evaluation of the Edwards Lifesciences Percutaneous Mitral Annuloplasty System for the Treatment of Mitral Regurgitation). JACC Cardiovasc Interv 2011;4:115-22. [PubMed]
- Siminiak T, Dankowski R, Baszko A, et al. Percutaneous direct mitral annuloplasty using the Mitralign Bident system: description of the method and a case report. Kardiol Pol 2013;71:1287-92. [PubMed]
- Palacios IF, Condado JA, Brandi S, et al. Safety and feasibility of acute percutaneous septal sinus shortening: first-in-human experience. Catheter Cardiovasc Interv 2007;69:513-8. [PubMed]
- Grossi EA, Patel N, Woo YJ, et al. Outcomes of the RESTOR-MV Trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve). J Am Coll Cardiol 2010;56:1984-93. [PubMed]





